Abstract
Objective: This study examines psychological well-being levels among adolescents with polycystic ovary syndrome (PCOS) by levels of biochemical markers used for their diagnoses.
Methods: A cross-sectional study involved 45 adolescent females with PCOS at a pediatric endocrinology outpatient clinic. Data, including demographics, clinical exams, and lab results, were recorded at enrollment. Initial blood samples included metabolic and hormonal markers. Mental health was assessed using the Depression Anxiety Stress Scale (DASS)-42 questionnaire.
Results: The study provides valuable insights into the possible metabolic and hormonal influences on mental health in adolescents with PCOS, detecting that total testosterone (TT) exhibits high sensitivity for depression, while aspartate transaminase (AST) presents notable specificity for stress. Anxiety did not show a significant link with laboratory data.
Conclusion: TT exhibits high sensitivity for depression, while AST presents notable specificity for stress. Both markers suggest diagnostic potential in their respective categories, necessitating further research for validation.
Keywords: adolescents, polycystic ovary syndrome, mental health, depression, stress
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a hormonal disorder in women of reproductive age. Its exact cause remains elusive, but it is understood to be multifactorial, stemming from genetic and environmental influences. Factors such as heredity, hormonal imbalance, obesity, and insulin resistance are associated with an elevated risk of PCOS, though they do not directly induce the condition.1 PCOS presents a spectrum of immediate and long-term complications, ranging from infertility and endometrial anomalies (hyperplasia and cancer) to metabolic challenges such as type 2 diabetes, gestational diabetes, pregnancy-induced hypertension, non-alcoholic fatty liver disease, dyslipidemia, and obesity, alongside manifestations like acne and psychological disturbances including depression and anxiety.2 Given the breadth of these potential complications, early diagnosis, regular monitoring, and appropriate management of PCOS are crucial.
Numerous studies exist regarding preventing, detecting, and monitoring complications associated with PCOS. Clinical practice appears to prioritize the evident complications of PCOS, including metabolic, cardiovascular, and gynecological concerns. Although PCOS is primarily a hormonal and metabolic disorder, it can also significantly affect a woman’s mental health. The psychological dimensions of the disease present potential risks, which may be overlooked unless the patient exhibits significant distress or impairment in this domain. Multiple studies have reported a higher prevalence of psychological disorders and symptoms among women with PCOS than those without.3,4 It is well-documented that women with PCOS generally have higher rates of depression, anxiety, and stress than the general population.5 This can be attributed to hormonal fluctuations, the cumulative stress of dealing with symptoms, and the challenges of managing a chronic health condition. It is believed that the hormonal imbalances inherent in PCOS might directly influence mood and psychological well-being. For example, elevated androgens might be associated with mood disturbances.6
Various measurement tools have been used to detect these psychological aspects of PCOS in patients, including other self-report questionnaires and clinical interviews.4 Although self-report questionnaires have their uses, they may be insufficient in accurately assessing mental health. On the other hand, the evaluation process of these patients by mental health professionals is only when the patient reports a psychological complaint. During the diagnosis and follow-up of PCOS, a number of blood laboratory values are examined. However, no marker is used to determine the psychiatric or psychological effects of the disease. Thus, the complication of psychological involvement in PCOS remains clinically and laboratory in the background. This study sought to elucidate the relationship between the parameters employed as diagnostic adjuncts for PCOS and patients’ psychological well-being, suggesting that the extant blood sample results might offer insights into the potential psychological ramifications of the disease.
MATERIALS AND METHODS
Adolescents diagnosed with PCOS were recruited for the study after informed consent was obtained from both them and their parents.
Selection and Identification of Cases
This study enrolled 45 adolescent females with PCOS, selected through continuous sampling after referral to the Health Sciences University Bursa Yüksek Ihtısas Education and Researh Hospital pediartic endocrinology outpatient clinic. Based on the Rotterdam criteria of 2003, PCOS was diagnosed in the patients.2 Exclusion criteria included metabolic syndrome, conventional medication (e.g., oral contraceptives, metformin, and medication’s effects on sleep or mental status), chronic disease history, alcohol consumption, pregnancy, and lactation.
Data collection
Clinical and anthropometric data were collected during enrollment. Researchers recorded demographic information, clinical examination results (height, weight, Body mass index (BMI), hyperandrogenism features), and laboratory test results in a questionnaire for each patient. Income levels were determined based on the official starvation and poverty limits of 2023.
Anthropometric measurements
Using a daily calibrated stadiometer (Seca 703, SecaGmBH&Co Kg, Hamburg, Germany) that ensures accuracy of 0.1 cm for height and 0.1 kg for weight, patients were assessed for their body weight and height while dressed in their underwear and without shoes or outer clothing. The BMI was computed as the weight in kilograms divided by the square of the height in meters. A BMI over the 85th percentile was classified as overweight, while one above the 95th percentile was classified as obese.7
Physical examination parameters
Blood pressure was measured twice at rest with a 15-minute interval to screen all patients for hypertension. Any patients with hypertension (n=2) were excluded from the study.
The modified Ferriman-Gallwey (mFG) scoring system was used to diagnose hirsutism, evaluating hair growth in nine regions (upper lip, chin, chest, back, waist, upper abdomen, lower abdomen, arm, and thigh) and scoring each region from 0 to 4 according to the terminal hair growth rate. The total score is calculated, and females with an FG score of 8 or higher are considered hirsute.8
Laboratory-derived metrics
During their first visit to the endocrinology clinic, blood samples were taken to diagnose PCOS. The samples included metabolic (fasting glucose, serum insulin, alanine transaminase (ALT), aspartate transaminase (AST), lipids; triglycerides (TG), high-density cholesterol (HDL-C), low-density cholesterol (LDL-C), and total cholesterol (TC) and hormonal (luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (E2), prolactin (PRL), thyrotropin (TSH), free thyroxine (fT4), total and free testosterone, dehydroepiandrostenedione sulfate (DHEAS), Δ4-androstenedione, and 17 OH progesterone) measurements. The insulin resistance index was calculated using the homeostatic model assessment–insulin resistance (HOMA-IR) formula, equal to (fasting plasma glucose x fasting serum insulin) divided by 22.5.9
Mental health assessment
The Depression Anxiety Stress Scale (DASS)-42 assessed participants’ mental health. The DASS-42 is widely used in clinical and non-clinical populations, including adolescents. The method was developed by Lovibond and Lovibond in 1995 and adapted for Turkish use by Bilgel and Bayram in 2010.10,11 It displayed high levels of internal consistency reliability, with Cronbach’s alpha coefficients of .96 for depression, .89 for anxiety, and .93 for stress. In the Turkish version of the DASS-42, the Cronbach alpha coefficients for depression, anxiety, and stress were .92, .86, and .88, respectively. This self-report questionnaire includes 42 items to measure depression, anxiety, and stress. Respondents rate 14 items on a 4-point severity/frequency scale, with options ranging from ‘0’ (not suitable) to ‘3’ (completely suitable) to indicate how often they experienced each state over the past week. The scale measures depression, anxiety, and stress, with scores ranging from 0 to 42 for each sub-dimension. These categories express the severity of the conditions: normal, mild, moderate, severe, and very severe. The Anxiety scale assesses autonomic arousal, skeletal muscle effects, situational anxiety, and subjective experience of anxious affect. For anxiety, scores of 8-9, 10-14, 15-19, and ≥20 are mild, moderate, severe, and severe, respectively. The Stress scale is sensitive to levels of chronic non-specific arousal, assessing difficulty relaxing, nervous arousal, and being easily upset/agitated, irritable/over-reactive, and impatient. Mild stress is 15-18, moderate stress is 19-25, severe stress is 26-33, and ≥34 is extremely severe. The Depression scale assesses dysphoria, hopelessness, devaluation of life, self-deprecation, lack of interest/involvement, anhedonia, and inertia. Mild depression is 10-13, moderate depression is 14-20, severe depression is 21-27, and very severe depression is ≥28. This study compared patients with normal scores with those outside the normal range.
Ethical approval
The study was approved by the local ethics committee of Bursa Yüksek İhtisas Training and Research Hospital Clinical Research Ethics Committee and conducted in accordance with the principles of the Declaration of Helsinki (Ethical approval number: 2011-KAEK-25 2023/02-16). Adolescents diagnosed with PCOS were recruited for the study after informed verbal and written consent was obtained from both them and their parents.
Statistics
The Shapiro-Wilk test examined the data to determine whether it presented a normal distribution. The results were presented as mean ± standard deviation, median (minimum-maximum), or frequency and percentage. Mann-Whitney U test was used to compare the two groups. The Pearson correlation coefficient was calculated for the relationship between variables. Statistically, the significance level was accepted as α=0.05. Statistical analyses were performed with IBM SPSS ver.28.0 (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp.).
Receiver operating characteristics (ROC) curve analysis was performed to determine optimal cut-off values for the significant variables of triglyceride according to depression and stress. The area under the curve (AUC) with a confidence interval, sensitivity, and specificity values were given for optimal cut-off value. MedCalc Statistical Software version 22.006 was used for ROC analysis (MedCalc® Statistical Software version 22.006 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2023).
RESULTS
Demographics and clinical features
The study involved 45 PCOS adolescents with a mean age of 16.17±1.4 years. The BMI of the participants was 27.02±6.04. Twelve (26.6%) patients were obese, and 16 (35.6%) were overweight. The sociodemographic characteristics of the study subjects are summarized in Table 1.
| Table 1. Socio-demographics and characteristics of the patients | ||
|---|---|---|
| NVD; normal vaginal delivery, C/S; cesarean section, BMI; body mass index | ||
| Age, year | 16.17±1.4 | |
| Delivery mode |
NVD C/S |
29 (64.4%) 16 (35.6%) |
| BMI, kg/m2 | 27.02±6.04 | |
| BMI sds | 1.48±1.63 | |
|
Underweight Normal Overweight Obese |
2 (4.4%) 15 (33.3%) 16 (35.6%) 12 (26.6%) |
|
| Socio-economic status |
low medium good |
2 (4.4%) 40 (88.9%) 3 (6.7%) |
| Presenting Symptoms |
menstrual irregularity weight gain acne hair growth |
23(51.1%) 8(17.8%) 2(4.4%) 12(26.7%) |
Presenting symptoms
Patients applied to the hospital with four different symptoms. The most common complaint of the patients at hospital admission was menstrual irregularity (n=23, 51.1%). The others were as follows: weight gain (n=8, 17.8%), acne (n=2, 4.4%), and hair growth (n=12, 26.7%).
Psychological assessments
Participants were divided into two groups based on their scores on the DASS-42 subscales: normal range (NR) and high score (HS). The groups were then compared based on their laboratory values. Table 2 displays participants’ laboratory values and DASS-42 subscale results.
| The values are shown as mean ± standard deviation. NR; normal range, HS; high score (indicative of higher depression, anxiety, or stress). The p-values indicate the statistical significance of the differences between the NR and HS groups for each condition (depression, anxiety, and stress). A p-value less than 0.05 is considered statistically significant. BMI; body mass index (kg/m2), SD; standard deviation, WC; waist circumference (cm), FG; fasting glucose (mg/dl), FI; fasting insülin (µU/L), HOMA-IR homeostasis model assessment insulin resistance index, TC; total cholesterol (mg/dl), LDL-C; low-density lipoprotein cholesterol (mg/dl), HDL-C; high-density lipoprotein cholesterol (mg/dl), TG; triglyceride (mg/dl), TSH; thyroid stimulating hormone (mIU/L), fT4; free thyroxine (ng/dL), FSH; follicle-stimulating hormone (µU/ml), LH; luteinizing hormone (µU/ml), E2; Estradiol (pg/ml), TT; total testosterone (ng/dl), PRL; prolactin (ng/mL), DHEAS; dehydroepiandrosterone sulfate (µg/dl), 17 OH PG; 17 OH progesterone (ng/ml), Androstenedione; ng/ml, ALT; alanine transaminase (IU/L), AST; aspartate transaminase (IU/L), mFGS; modified Ferriman-Gallwey score. | |||||||||
| Table 2. Laboratory profiles of PCOS patients according to the Depression Anxiety Stress Scale | |||||||||
| depression | anxiety | stress | |||||||
| NR | HS (≥9) | p | NR | HS (≥8) | p | NR | ≥15 (HS) | p | |
| n (%) | 19 (43.2) | 25 (56.8) | 17 (37.8) | 28 (62.2) | 16 (37.2%) | 27 (62.85) | |||
| BMI | 25.25±7.6 | 27.16±6.5 | 0.507 | 25.12±5.2 | 27.11±7.8 | 0.237 | 25.79±5.9 | 26.44±7.7 | 0.615 |
| BMI, sd | 1.46±1.4 | 1.48±1.8 | 0.722 | 1.02±1.5 | 1.76±1.6 | 0.111 | 1.13±1.6 | 1.62±1.6 | 0.327 |
| WC | 82.47±22.2 | 88.5±16.6 | 0.400 | 79.68±21.1 | 89.71±16.9 | 0.128 | 84.69±15.2 | 86.06±21.6 | 0.669 |
| FG | 85.89±8.9 | 85.72±7.1 | 0.859 | 84.76±8.2 | 86.14±7.7 | 0.582 | 86.88±8.7 | 85.26±7.5 | 0.615 |
| FI | 10.61±5.5 | 14.99±10.1 | 0.201 | 11.72±4.7 | 13.81±10.2 | 0.861 | 10.96±5.2 | 14.09±10.1 | 0.400 |
| HOMA-IR | 2.46±1.2 | 3.08±1.9 | 0.462 | 2.46±1.19 | 2.99±1.8 | 0.708 | 2.49±1.2 | 2.95±1.8 | 0.660 |
| TC | 174.58±28.7 | 173.4±40.0 | 0.767 | 171.06±34.8 | 175.11±35.5 | 0.665 | 176.25±36.2 | 172.59±35.9 | 0.651 |
| LDL-C | 96.08±24.7 | 92.25±37.4 | 0.368 | 93.39±25.5 | 94.01±35.7 | 0.888 | 93.16±30.8 | 94.3±34.2 | 0.763 |
| HDL-C | 59.22±16.5 | 51.35±12.4 | 0.107 | 60.61±16.8 | 50.86±11.9 | 0.075 | 61.94±14.6 | 51.07±13.3 | 0.011 |
| TG | 85.48±37.6 | 116.48±56.7 | 0.045 | 87.07±43.7 | 113.68±52.8 | 0.090 | 75.58±31.9 | 116.19±53.1 | 0.008 |
| TSH | 1.94±0.9 | 1.51±0.6 | 0.196 | 1.56±0.6 | 1.77±0.8 | 0.460 | 1.52±0.5 | 1.75±0.8 | 0.505 |
| fT4 | 1.04±0.1 | 0.98±0.07 | 0.030 | 1.01±0.09 | 1±0.09 | 0.550 | 1±0.08 | 1.02±0 | 0.847 |
| FSH | 5.13±1.4 | 4.42±1.5 | 0.083 | 4.51±1.6 | 4.92±1.5 | 0.276 | 4.53±1.6 | 4.84±1.4 | 0.386 |
| LH | 6.94±5.01 | 5.03±4.1 | 0.188 | 6.21±5.3 | 5.53±4 | 0.944 | 7.25±5.9 | 4.99±3.5 | 0.327 |
| E2 | 42.69±21.1 | 35±14.1 | 0.055 | 38.47±22.5 | 37.79±14.2 | 0.752 | 41.13±22.2 | 37.16±14.7 | 0.352 |
| TT | 56.26±28.7 | 40±19.9 | 0.027 | 52.12±30.3 | 44.68±21 | 0.517 | 49.13±25.6 | 47.1±26.5 | 0.746 |
| PRL | 18.92±14.0 | 14.55±6 | 0.722 | 18.22±14.1 | 15.06±7.1 | 0.752 | 16.88±11 | 15.86±10.2 | 0.716 |
| DHEAS | 338.74±177.2 | 257.06±104.8 | 0.166 | 337.61±165.3 | 266.26±123.5 | 0.143 | 303.71±117 | 286.71±163.2 | 0.498 |
| 17 OH PG | 0.49±0.2 | 0.47±0.3 | 0.441 | 0.58±0.3 | 0.41±0.2 | 0.061 | 0.47±0.2 | 0.48±0.3 | 0.497 |
| Androstenedione | 1.25±0.5 | 1.06±0.6 | 0.168 | 1.38±0.6 | 0.99±0.5 | 0.055 | 1.25±0.5 | 1.04±0.6 | 0.152 |
| LH_FSH_ratio | 1.34±0.7 | 1.16±0.9 | 0.222 | 1.3±0.8 | 1.18±0.9 | 0.426 | 1.52±0.9 | 1.06±0.8 | 0.067 |
| AST | 16.00±4.4 | 16.08±4.7 | 0.962 | 14.71±3.3 | 16.86±4.9 | 0.244 | 13.44±2.5 | 17.33±4.7 | 0.004 |
| ALT | 14.16±8.0 | 19.00±11.5 | 0.089 | 13.00±5.7 | 19.04±11.7 | 0.076 | 12.63±6 | 18.70±11.2 | 0.035 |
| mFGS ≥8 | 12 (66.7) | 13 (54.2) | 0.414 | 9 (56.3) | 16 (59.3) | 0.847 | 8 (53.3) | 17 (65.4) | 0.446 |
Body mass index (BMI)
There was no statistically significant difference in BMI among groups of depression, anxiety, or stress based on the provided p-values (all > 0.05) (Table 2).
Modified Ferriman Gallwey Score (mFGS)
Of the group, 25 patients (55.55%) had a mFGS score of 8 and above. However, when contrasting the results of patients with mFGS scores below 8 to those scoring eight and above, no statistically significant differences emerged for depression (p=0.46), anxiety (p=0.87), or stress (p=0.72). Further evaluation of patients with mFGS scores ≥8 similarly revealed no significant correlation, as detailed in Table 2.
Blood lipid profiles (TC, LDL-C, HDL-C, and TG)
TC and LDL-C did not exhibit a statistically significant difference in evaluating lipid metrics, as depicted in Table 2. Lower HDL-C levels were associated with higher stress scores (p=0.011). Concurrently, there was a notable elevation in TG levels corresponding to heightened stress and depression scores (p-values < 0.05). Contrastingly, nonHDL-C did not present any variations across the respective sub-scales (Table 2).
The TG levels of 38 patients were within the normal range, defined by an upper limit of 150 mg/dl. No statistically significant difference was found when the sub-scale results of patients with blood TG values below and above 150 mg/dl were compared (p= 0.17 for depression, p=0.07 for anxiety, and p= 0.065 for stress).
Logistic regression analysis
Logistic regression analysis evaluated odds ratios (ORs) for two stress-related variables (TG and HDL-C). The result suggests that for every one-unit increase in TG, the odds of stress increase by 3.1%. HDL-C levels were inversely associated with stress, which indicated that for every one-unit increase in HDL-C, the odds of stress decreased by 6.1% (Table 3).
| Table 3. Logistic regression analysis of Laboratory profiles in relation to stress and ROC curve analysis for depression and stress variables | ||||||
|---|---|---|---|---|---|---|
| p | OR | 95% CI for OR | ||||
| OR; odds ratio, Cl; confidence interval, TG; triglyceride, HDL-C; high-density cholesterol, AST; aspartate transaminase, ALT; alanine transaminase, TT; total testosteron, fT4; free thyroxine | ||||||
| TG (mg/dl) | 0.028 | 1.045 | 1.005 | 1.088 | ||
| HDL-C (mg/dl) | 0.037 | 0.916 | 0.844 | 0.995 | ||
| AST (IU/L) | 0.023 | 1.839 | 1.088 | 3.106 | ||
| ALT (IU/L) | 0.196 | 0.843 | 0.652 | 1.092 | ||
| Criterion | AUC | 95% Cl | p | Sensitivity (%) | Specificity (%) | |
| Depression | ||||||
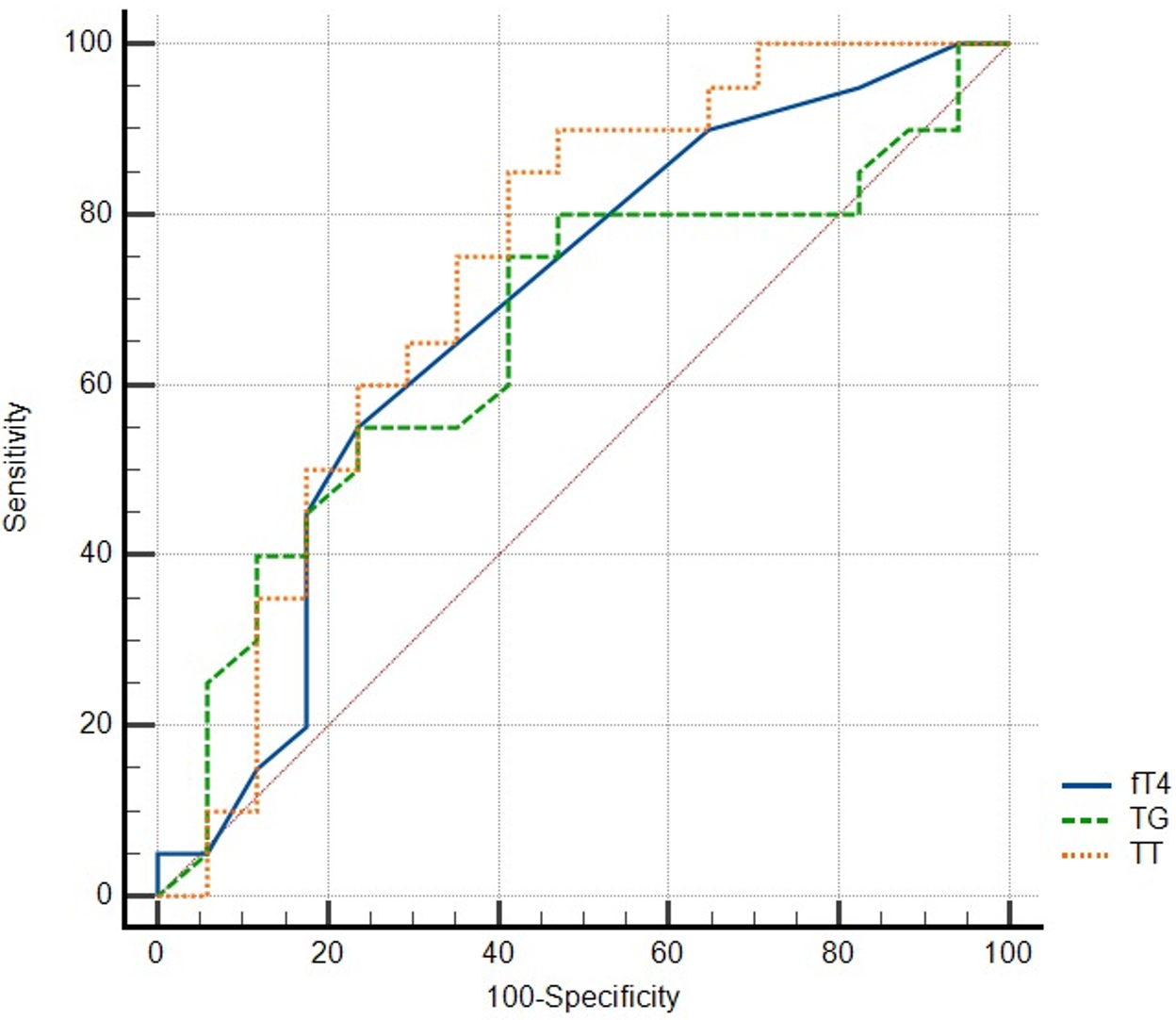
| TG | >81 | 0.678 | 0.520-0.811 | 0.0327 | 76 | 57.89 |
| TT | ≤58 | 0.704 | 0.539-0.838 | 0.0180 | 85.71 | 52.63 |
| fT4 | ≤0,99 | 0,696 | 0.533-0.830 | 0.0252 | 58.33 | 76.47 |
| Stress | ||||||
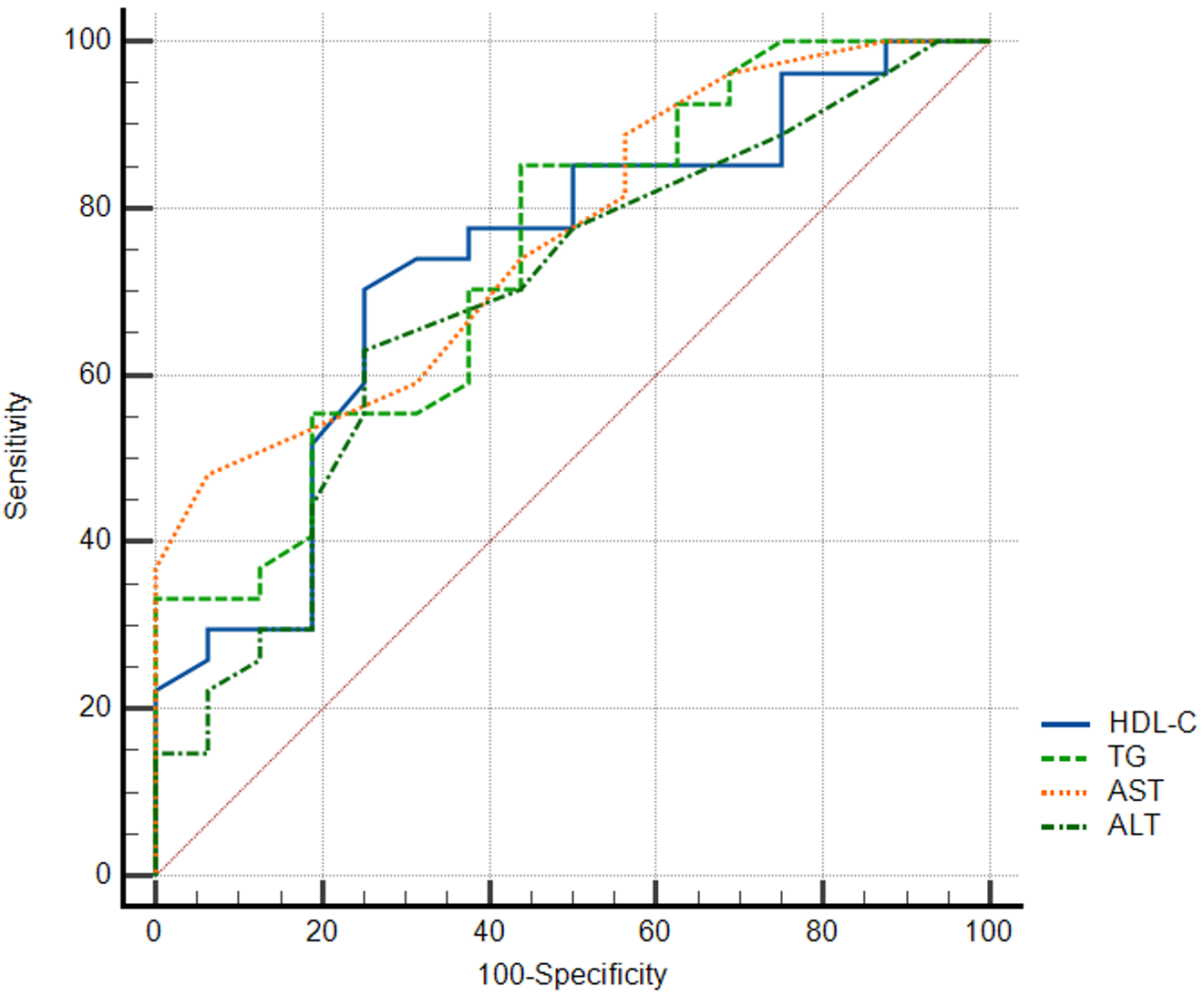
| TG | >74 | 0.744 | 0.588-0.865 | 0.0017 | 85.19 | 56.25 |
| HDL_C | ≤52 | 0.734 | 0.577-0.857 | 0.0038 | 70.37 | 75 |
| ALT | >12 | 0.693 | 0.534-0.825 | 0.0221 | 62.96 | 75 |
| AST | >16 | 0.766 | 0.612-0.882 | 0.0002 | 48.15 | 93.75 |
ROC analysis of depression and stress diagnostic tools
ROC curve analysis of variables for depression and stress is depicted in Figure 1 and Figure 2, respectively. Table 3 presents the ROC curve analysis for various biochemical variables in relation to depression and stress. In terms of depression, TT and TG demonstrated substantial areas under the curve (AUCs) with significance, where TT particularly showcased high sensitivity at 85.71%. For stress markers, AST stood out with the highest AUC of 0.766, signifying its potential diagnostic power, and it also showed an impressive specificity of 93.75%. The results suggest that these specific biochemical markers, especially AST and TT, may be significant in understanding and diagnosing depression and stress.
Examining the multivariate effects of the significant univariate variables from Table 2 on depression, only TT was significant (p=0.027, 0R=0.962).
Thyroid-related measures (TSH and fT4)
TSH levels did not differ significantly between groups, while fT4 levels significantly decreased with higher depression scores (p = 0.030).
Hormonal measures (FSH, LH, E2, TT, PRL, DHEAS, 17 OH PG, Δ4-androstenedione, and LH/FSH ratio)
There were no significant differences in most measures among the groups, except for total testosterone level, which showed a significant decrease for higher depression scores (p = 0.027) (Table 2).
DISCUSSION
This research focuses on the potential link between metabolic and hormone profiles in adolescents with PCOS and their mental health, as quantified by the DASS-42.
Relationship between common characteristics of PCOS and mental health BMI
The relationship between BMI, PCOS, and mental health is complex. Patients with PCOS generally have an increased BMI. Elevated BMI, which indicates overweight or obesity, is associated with an increased risk of various psychological issues, irrespective of PCOS. Being overweight and obese is linked with body image concerns, lower self-esteem, and a higher prevalence of depression, among other concerns.12 Some research suggests that women with PCOS and a higher BMI have an increased prevalence of depressive and anxiety symptoms compared to women with PCOS and a normal BMI.7 In the current research, most participants had elevated BMI, placing them in the overweight and obese category, typical of PCOS presentations. However, there was no significant correlation between BMI and mental health indicators such as depression, anxiety, or stress, suggesting that participants’ weight alone did not notably impact their mental health outcomes.
Hirsutism defined with mFGS
The mFGS is used to evaluate the degree of hirsutism in patients, a common symptom of PCOS. In this study, over half (55.55%) of the sampled adolescents with PCOS had an mFGS score of 8 or above, suggesting a substantial prevalence of hirsutism. Historically, higher mFGS scores have often been associated with elevated psychosocial distress.13 The intuitive link is that the aesthetic concerns and stigma of hirsutism may contribute to these psychosocial effects. However, intriguingly, the current study found no statistically significant difference in levels of depression, anxiety, or stress between patients with mFGS scores below eight and those with scores of 8 or above. This result in our study may be related to the fact that body hair is no longer a concern compared to previous years with the developing technology.
The connection between laboratory characteristics of PCOS and mental health
TG and HDL-C
Among blood lipid profiles, TG was a significant predictor for both stress and depression in the present study. TG levels are primarily discussed in the context of cardiovascular health, as elevated levels can serve as a risk factor for heart disease. However, recent research has also begun to explore the potential associations between lipid levels, including triglycerides, and various psychological conditions.14 In most (84%) participants, the TG level was below 150 mg/dl, considered the pathological laboratory limit. Previous studies have shown a potential link between high TG levels and depression14, although the nature and direction of this association remain unclear. The mechanisms underlying this relationship have yet to be thoroughly explored. However, it is suggested that systemic inflammation, which can be associated with elevated TG, might play a role in depression. Our study observed that depression and stress scores increased as TG increased, although within normal limits. Although still within the normal range, we saw that the lower HDL-C level affected the stress scores and contributed negatively to this. Higher levels of TG have been associated with cardiovascular risks and other metabolic problems, so it is interesting to note that in our sample, it was also associated with mental health within the normal range. In the current study, the ROC analysis provided cut-off values for TG that might be used to predict the presence of depression or stress. The areas under the curve (AUC) for both were reasonably good (though not outstanding), suggesting that this cut-off might serve as a useful, albeit not definitive, biomarker. The odds of experiencing stress increased by 3.1% for every one-unit increase in TG.
Conversely, an increase in HDL-C led to a decrease in stress odds by 6.1%. This suggests that lipid profiles, particularly TG and HDL-C, could play a role in mental health, at least in terms of stress. Lipids, especially in the context of metabolic syndrome, can influence inflammatory pathways in the body. Chronic inflammation is increasingly recognized as a factor in several psychiatric conditions, including depression and anxiety disorders.15 On the other hand, HDL-C has anti-inflammatory and antioxidant properties that might offer some neuroprotective effects. HDL-C helps stabilize cell membranes, which could stabilize mood.16 Given the brain’s heavy reliance on cholesterol, balanced HDL-C levels might affect optimal brain health. Some studies have suggested that higher HDL-C levels might be protective against cognitive decline and dementia. Lower HDL-C levels have been observed in some people with depression and anxiety.17 While the association exists, causality is still a topic of debate. We highlighted that HDL-C levels were inversely associated with stress, implying a potential protective role. One theory is that HDL-C’s anti-inflammatory and antioxidant properties help buffer against some adverse effects of chronic stress on the brain. As PCOS is a chronic disease, we deduced that even within the normal range, TG may potentially boost inflammation, whereas HDL-C could act as a safeguard against inflammation. Being within the normal range for TG does not necessarily imply a significant risk for mental health issues. However, metabolic health is a part of the broader picture of mental health. More extensive cohort studies and more focused research are needed to provide a more definitive answer about the connection between “normal” TG levels and mental health.
fT4
In the current research, low fT4 levels were significantly associated with higher depression scores. The thyroid plays a critical role in metabolism and mood, so imbalances can have various systemic effects. Both low (hypothyroidism) and high (hyperthyroidism) levels of thyroid hormones can be associated with mental health. While the most commonly discussed thyroid hormone in relation to depression is thyroid-stimulating hormone (TSH), altered free T4 levels can also indicate underlying thyroid dysfunction.18 The relationship between thyroid function and PCOS is complex and still a subject of ongoing research. In some PCOS patients, there may be alterations in thyroid hormone levels.19 PCOS is often associated with metabolic syndrome, which includes hypertension, dyslipidemia, insulin resistance, and obesity. Changes in metabolism and these associated conditions might indirectly influence thyroid hormone regulation. Many factors affect the levels and effects of free T4 on mental health, including genetics, autoantibodies (as in Hashimoto’s thyroiditis or Graves’ disease), other medical conditions, and medications. Additionally, the absolute value of free T4 does not always matter. The balance between free T4, free T3, and TSH, as well as how these hormones interact with various systems in the body, can influence mental health outcomes.20
TT
In our study, examining the multivariate effects of the significant univariate variables on depression, only TT showed a significant association with depression, revealing a decrease in increased depression.
Testosterone has various effects on the brain, influencing mood, cognition, and behavior. Testosterone might also influence the brain’s stress response.21 Some studies suggest elevated testosterone might contribute to mood dysregulation, but the evidence is inconsistent across all research.22 While high testosterone levels can negatively impact mood, evidence suggests testosterone might have neuroprotective effects.23 Testosterone can influence the serotonergic system in the brain, which plays a vital role in mood regulation.24
Moreover, testosterone may help in reducing the risk of certain neurodegenerative conditions. Some studies suggest that hormone replacement therapy is effective in reducing depressed mood in menopausal women.25 However, the relevance of these findings to PCOS patients is still a matter of discussion. It is crucial to understand that while testosterone might play a role in the mental health of women with PCOS, it is just one piece of a complex puzzle. Genetics, lifestyle factors, other hormonal imbalances, and the psychosocial effects of living with a chronic condition like PCOS influence mental health. In conclusion, while testosterone might have some protective effects on the brain and mental health, its role in PCOS is nuanced and can vary among individuals. There are potential benefits and drawbacks to elevated testosterone levels in the context of PCOS and mental health, and more research is needed to understand this relationship fully.
ALT and AST
Our findings suggest that AST has greater specificity in indicating stress, but its sensitivity is lower than ALT. However, both enzymes present potential as stress biomarkers, with AST demonstrating extreme discriminative power. Elevated levels traditionally suggest potential liver damage or inflammation. It is possible that stress, through various mechanisms, impacts liver function or exacerbates pre-existing liver conditions, leading to elevated ALT and AST.26 Remarkably, ALT and AST are effective in the stress scale, albeit within normal limits, since PCOS patients with metabolic syndrome and cases with ALT and AST values outside the normal range were not included in our study. Even if AST and ALT are within normal ranges, it does not rule out metabolic stress or early metabolic disturbances in PCOS patients. PCOS patients often have underlying insulin resistance, which might not immediately reflect in elevated liver enzymes but could still represent a form of metabolic stress. In summary, while normal levels of AST and ALT are reassuring regarding liver health, they do not directly provide insights into the various forms of stress that a PCOS patient might be experiencing. It is crucial to have a multifaceted approach when evaluating stress and its impacts on PCOS patients.
CONCLUSION
The study provides valuable insights into the possible metabolic and hormonal influences on mental health in adolescents with PCOS, detecting that TT exhibits high sensitivity for depression, while AST presents notable specificity for stress. It should be kept in mind that the correlation between PCOS and mental health is complex and may be influenced by various factors, including hormonal imbalances, symptoms such as weight gain or hirsutism, and the psychological impact of dealing with a chronic illness. The associations discovered offer potential avenues for clinical interventions and further research.
Limitations
It is crucial to consider potential limitations. The study has a relatively small sample size, potentially limiting its statistical power and generalizability to the broader population of adolescents with PCOS. Participants were sourced from a single pediatric endocrinology outpatient clinic, which may introduce selection bias. As a cross-sectional study, it provides a snapshot at one point without elucidating causality or temporal relationships between variables. Unmeasured confounders, like lifestyle or dietary habits, could influence the results despite controlling for certain factors. Moreover, solely using the DASS-42 questionnaire for mental health assessment, though recognized, may not capture the full spectrum of psychological well-being that could be achieved with multiple tools.
Acknowledgments
We express our gratitude to all the adolescents who took part in the survey and to the hospital staff for their support and cooperation. Additionally, the authors would like to thank Güven Özkaya, Biostatistics, Bursa Uludağ University, for kindly reviewing statistics.
Ethical approval
This study has been approved by the Bursa Yüksek İhtisas Training and Research Hospital Clinical Research Ethics Committee (approval date 22.02.2023, number 2011-KAEK-25 2023/02-16). Adolescents diagnosed with PCOS were recruited for the study after informed verbal and written consent was obtained from both them and their parents.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Diamanti-Kandarakis E, Piperi C. Genetics of polycystic ovary syndrome: searching for the way out of the labyrinth. Hum Reprod Update. 2005;11:631-43. https://doi.org/10.1093/humupd/dmi025
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-7. https://doi.org/10.1093/humrep/deh098
- Dokras A, Clifton S, Futterweit W, Wild R. Increased risk for abnormal depression scores in women with polycystic ovary syndrome: a systematic review and meta-analysis. Obstet Gynecol. 2011;117:145-52. https://doi.org/10.1097/AOG.0b013e318202b0a4
- Dokras A, Clifton S, Futterweit W, Wild R. Increased prevalence of anxiety symptoms in women with polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2012;97:225-30.e2. https://doi.org/10.1016/j.fertnstert.2011.10.022
- Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2017;32:1075-91. https://doi.org/10.1093/humrep/dex044
- Ethirajulu A, Alkasabera A, Onyali CB, et al. Insulin resistance, hyperandrogenism, and its associated symptoms are the precipitating factors for depression in women with polycystic ovarian syndrome. Cureus. 2021;13:e18013. https://doi.org/10.7759/cureus.18013
- Centers for Disease Control and Prevention (CDC). About Child & Teen BMI. Available at: https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html
- Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440-7. https://doi.org/10.1210/jcem-21-11-1440
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-9. https://doi.org/10.1007/BF00280883
- Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33:335-43. https://doi.org/10.1016/0005-7967(94)00075-u
- Bilgel N, Bayram N. Turkish version of the Depression Anxiety Stress Scale (DASS-42): psychometric properties. Archives of Neuropsychiatry. 2010;47:118-26.
- Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220-9. https://doi.org/10.1001/archgenpsychiatry.2010.2
- Deeks AA, Gibson-Helm ME, Paul E, Teede HJ. Is having polycystic ovary syndrome a predictor of poor psychological function including anxiety and depression? Hum Reprod. 2011;26:1399-407. https://doi.org/10.1093/humrep/der071
- van Reedt Dortland AKB, Giltay EJ, van Veen T, van Pelt J, Zitman FG, Penninx BWJH. Associations between serum lipids and major depressive disorder: results from the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry. 2010;71:729-36. https://doi.org/10.4088/JCP.08m04865blu
- Leighton SP, Nerurkar L, Krishnadas R, Johnman C, Graham GJ, Cavanagh J. Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol Psychiatry. 2018;23:48-58. https://doi.org/10.1038/mp.2017.205
- Sergi D, Zauli E, Tisato V, Secchiero P, Zauli G, Cervellati C. Lipids at the nexus between cerebrovascular disease and vascular dementia: The impact of HDL-cholesterol and ceramides. Int J Mol Sci. 2023;24:4403. https://doi.org/10.3390/ijms24054403
- Singh-Manoux A, Gimeno D, Kivimaki M, Brunner E, Marmot MG. Low HDL cholesterol is a risk factor for deficit and decline in memory in midlife: the Whitehall II study. Arterioscler Thromb Vasc Biol. 2008;28:1556-62. https://doi.org/10.1161/ATVBAHA.108.163998
- Hage MP, Azar ST. The link between thyroid function and depression. J Thyroid Res. 2012;2012:590648. https://doi.org/10.1155/2012/590648
- Sinha U, Sinharay K, Saha S, Longkumer TA, Baul SN, Pal SK. Thyroid disorders in polycystic ovarian syndrome subjects: a tertiary hospital based cross-sectional study from Eastern India. Indian J Endocrinol Metab. 2013;17:304-9. https://doi.org/10.4103/2230-8210.109714
- Bauer M, Goetz T, Glenn T, Whybrow PC. The thyroid-brain interaction in thyroid disorders and mood disorders. J Neuroendocrinol. 2008;20:1101-14. https://doi.org/10.1111/j.1365-2826.2008.01774.x
- McHenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Front Neuroendocrinol. 2014;35:42-57. https://doi.org/10.1016/j.yfrne.2013.09.001
- Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract. 2009;15:289-305. https://doi.org/10.1097/01.pra.0000358315.88931.fc
- Creta M, Riccio R, Chiancone F, Fusco F. Androgens exert direct neuroprotective effects on the brain: a review of pre-clinical evidences. Journal of Andrological Sciences. 2010;17:49-55.
- Höfer P, Lanzenberger R, Kasper S. Testosterone in the brain: neuroimaging findings and the potential role for neuropsychopharmacology. Eur Neuropsychopharmacol. 2013;23:79-88. https://doi.org/10.1016/j.euroneuro.2012.04.013
- Soares CN, Poitras JR, Prouty J. Effect of reproductive hormones and selective estrogen receptor modulators on mood during menopause. Drugs Aging. 2003;20:85-100. https://doi.org/10.2165/00002512-200320020-00001
- Tomasi D, Wang GJ, Wang R, et al. Association of body mass and brain activation during gastric distention: implications for obesity. PLoS One. 2009;4:e6847. https://doi.org/10.1371/journal.pone.0006847
Copyright and license
Copyright © 2025 The author(s). This is an open-access article published by Aydın Pediatric Society under the terms of the Creative Commons Attribution License (CC BY) which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.