Abstract
Objective: Type 1 diabetes (T1D) is a common, chronic, systemic disease in children and adolescents, and its incidence globally increases annually. This study aimed to determine the incidence, diagnostic features, and presentation characteristics of T1D in children and adolescents during the pandemic period in Bursa Province, Turkey.
Method: This study included children under 18 with newly diagnosed T1D who consulted the Pediatric Endocrinology clinics of 3 tertiary hospitals in the city center of Bursa between January 1, 2015, and December 31, 2022. Nine hundred twenty-one pediatric patients were included in the study. The patients were divided into four groups according to age: Group 1, 0-4.9 years; Group 2, 5-9.9 years; Group 3, 10-14.9 years; and Group 4, 15-18 years.
Results: Of the patients, 48.6% were female, and 51.4% were male. The median age at diagnosis was 9.23 years, with a significant age difference between genders. The highest incidence was observed in children aged 10-14.9 years. Moreover, two peaks were detected: 10-14.9 years in males and 5-9.9 years in females. The mean annual incidence was 3.8/100,000, peaking in 2017. During the COVID-19 pandemic, a temporary decline in diagnoses was noted, followed by an increase. Diabetic ketoacidosis (DKA) rates showed a significant rise over time, particularly in severe DKA cases during the pandemic.
Conclusion: This study provides the first comprehensive analysis of the rate of newly diagnosed T1D in children and adolescents in Bursa Province. Additionally, the study evaluated age, gender, and seasonal patterns of initial diagnoses, with global trends constantly. The incidence of T1D and the rate and severity of DKA at presentation were affected during the COVID-19 pandemic, causing lockdowns and healthcare avoidance.
Keywords: Bursa, COVID-19, incidence, ketoacidosis, type 1 diabetes, newly diagnosed type 1 diabetes, pandemic, trends
INTRODUCTION
Type 1 diabetes (T1D) is a common, chronic, systemic disease in children and adolescents. Although studies have reported that the incidence of T1D varies significantly among countries, a rising incidence of T1D has recently been detected in many populations.1-5 Also, seasonal and gender differences in the incidence of T1D have been reported.5-8 In contrast, some studies reported no change.9-12 During the COVID-19 pandemic, several studies have reported a significant increase in the initial diagnosis of T1D. As compared to previous years, the rate of diabetic ketoacidosis (DKA) was gradually elevated. Although studies have been conducted in recent years on the incidence and incidence trends of T1D in childhood in Turkey13-16, evaluating the COVID-19 pandemic or comparing it to before the pandemic is limited.17,18 In addition, no previous study has been conducted on the incidence of initially diagnosed diabetes and patient characteristics in Bursa Province.
This study aimed to determine the incidence of T1D in children and adolescents (under 18 years of age) in Bursa Province of Turkey between 2015 and 2022, to examine whether there was an increase in the incidence of new diagnoses over the years, to evaluate whether there was a difference in the presentation characteristics during the pandemic period, and to evaluate the age, type of diagnosis, gender, and season of diagnosis in children diagnosed with T1D.
MATERIAL AND METHODS
Patient selection
This study includes children under the age of 18 with newly diagnosed T1D who applied to the Pediatric Endocrinology clinics of 4 large regional hospitals in the city center of Bursa between January 1, 2015, and December 31, 2022, and whose T1D diagnosis was confirmed. However, one center could not be included in the study due to the incomplete number of diagnosed patients per year and its data, and the study was completed with the data of 3 centers. In addition, cases diagnosed at the age of 18 or older, those diagnosed with Type 2, monogenic, or neonatal diabetes despite being under the age of 18, and those diagnosed outside of Bursa were excluded from the study. When evaluating the diagnostic features, the patients were divided into four groups according to age: Group 1, 0-4.9 years; Group 2, 5-9.9 years; Group 3, 10-14.9 years; and Group 4, 15-18 years. Comparisons were made according to age and gender groups.
Laboratory
Serum glucose, blood gas, urine test, and blood/urine ketone values at the time of the first visit were examined from the files of all patients. In venous blood gas, pH values >7.30 and/or HCO3 >15 were classified as normal; 7.20-7.29 and/or HCO3 10-14.9 as mild acidosis, 7.10-7.19 and/or HCO3 5-9.9 as moderate acidosis and pH <7.09 and/or HCO3 <4.9 as severe acidosis. Normal values in urine density were accepted as 1003-1030, negative for glucose, and negative or trace amounts for ketone. A blood ketone level of less than 0.6 was considered ketone-negative.
Statistical analysis
Statistical analyses were performed using IBM SPSS 29.0.2.0 (IBM Corp. Released 2023. IBM SPSS Statistics for Windows, Version 29.0.2.0 Armonk, NY: IBM Corp.) statistical package program. Annual population sizes were obtained from the 2015-2022 Turkish census data from the address-based population registration system of the Turkish Statistical Institute. Pearson Chi-square and Fisher-Freeman-Halton tests were used in the analysis of categorical data. P < 0.05 was considered statistically significant.
Ethics
The study was approved by the Local Ethical Committee (Approval number: 2011-KAEK-25 2023/04-21) and conducted following the Declaration of Helsinki. The patient’s parents had signed informed written consent.
RESULTS
Nine hundred twenty-one patients (48.6% were female, and 51.4% were male) diagnosed with T1D from Pediatric Endocrinology clinics of three regional hospitals in the city center of Bursa were included in the study. The median age at diagnosis was 9.2 years (0.1-17.9 years), and the difference in age at diagnosis between the genders was statistically significant (9.00 years in females (0.1-17.8 years) and 9.80 years in males (0.1-17.9 years), p=0.035). Newly diagnosed T1D cases were the highest in males aged 10-14.9 years (n=161), followed by females aged 5-9.9 aged group in females (n=159), females aged 10-14.9 aged group in females (n=146), and 5-9.9 aged group in males (n=144) (Table 1).
| T1D: Type 1 diabetes. | |||
| Table 1. Distribution of initially diagnosed T1D patients by age group and gender | |||
|
|
|
|
|
| 0-4.9 years |
|
|
|
| 5-9.9 years |
|
|
|
| 10-14.9 years |
|
|
|
| 15-18 years |
|
|
|
| Total |
|
|
|
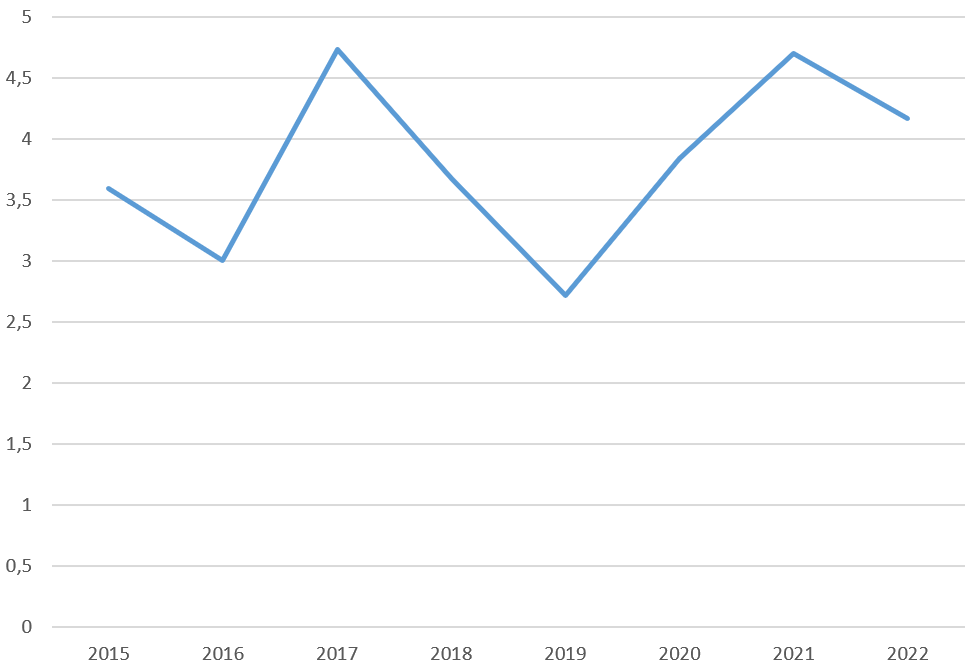
The mean annual incidence in children between 2015 and 2022 was 3.8 per 100.000. When evaluated by year, the highest incidence was found in 2017 (4.73/100.000). Figure 1 shows the incidence of newly diagnosed T1D by year.
The mean annual incidence between males (4.6%) and females (3%) was statistically significant only in 2020 (p=0.022); however, no difference was found for other years. In evaluating age groups, the mean annual incidence was 13.7/100,000 at the 0-4.9 age group, 16.0/100,000 at the 5-9.9 age group, 16.7/100,000 at the 10-14.9 age group, and 9.3/100,000 at the 15-18 age group. The highest incidence was in the 10-14.9 age group; the lowest incidence was in the 15-18 age group (9.3/100,000). As for evaluating seasons and months, winter and December were the most common seasons and months for newly diagnosed T1D through the years (Table 2).
| T1D: Type 1 diabetes. | |||||||||||
| Table 2. Distribution of newly diagnosed T1D cases by age group, gender, and season | |||||||||||
| Incidence |
|
|
|
|
|
|
|
|
|
|
|
| Gender | Female |
|
|
|
|
|
|
|
|
|
|
| Male |
|
|
|
|
|
|
|
|
|
||
| Age at diagnosis (years) | 0-4.9 |
|
|
|
|
|
|
|
|
|
|
| 5-9.9 |
|
|
|
|
|
|
|
|
|
||
| 10-14.9 |
|
|
|
|
|
|
|
|
|
||
| 15-18 |
|
|
|
|
|
|
|
|
|
||
| Female | 0-4.9 |
|
|
|
|
|
|
|
|
|
|
| 5-9.9 |
|
|
|
|
|
|
|
|
|
||
| 10-14.9 |
|
|
|
|
|
|
|
|
|
||
| 15-18 |
|
|
|
|
|
|
|
|
|
||
| Male | 0-4.9 |
|
|
|
|
|
|
|
|
|
|
| 5-9.9 |
|
|
|
|
|
|
|
|
|
||
| 10-14.9 |
|
|
|
|
|
|
|
|
|
||
| 15-18 |
|
|
|
|
|
|
|
|
|
||
| Season | Winter |
|
|
|
|
|
|
|
|
|
|
| Spring |
|
|
|
|
|
|
|
|
|
||
| Summer |
|
|
|
|
|
|
|
|
|
||
| Autumn |
|
|
|
|
|
|
|
|
|
||
At the beginning of the pandemic period and after the declaration of the pandemic and lockdown in Turkey (March 11, 2020), the rate of new diagnoses showed a significant decrease in April (0.161195), May (0.225673) and June (0.225673) compared to previous years and months [January 2020 (0.29), February (0.16) and March (0.42)], while an increase was detected in the following months, after the end of the lockdown of July (0.451346) and August (0.419107) compared to previous periods.
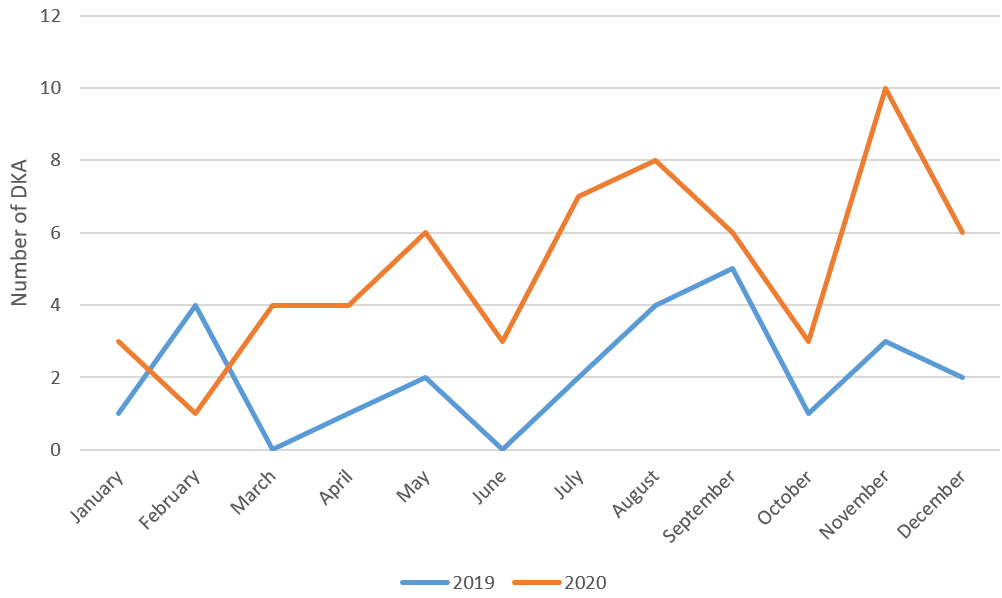
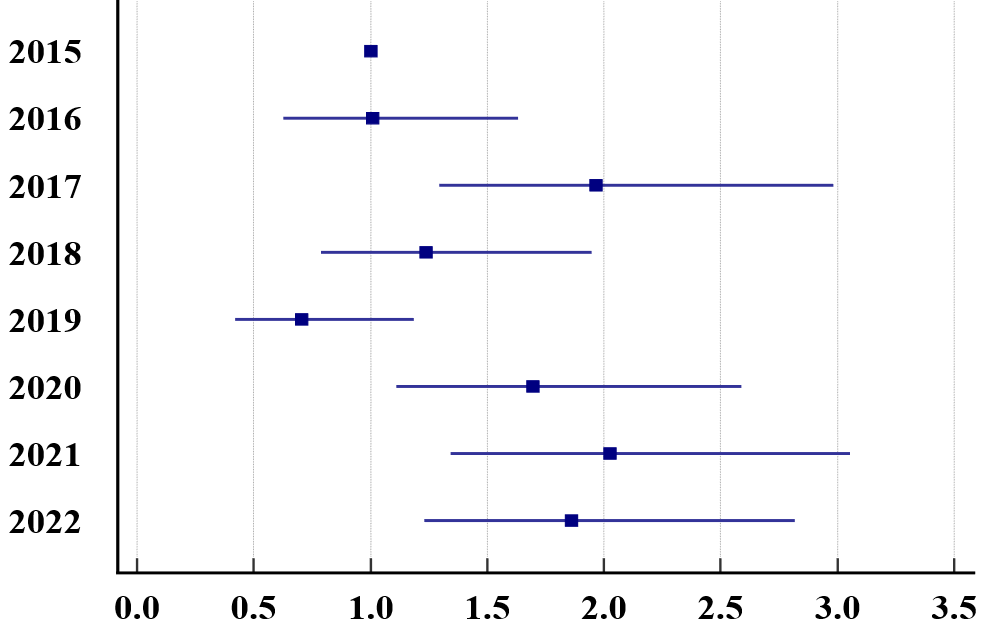
A statistically significant trend was found regarding DKA rates by year (p<0.001). While the DKA rate progressed similarly in 2016 compared to 2015, an increase was observed in 2017. While there was a decrease in 2019, there was a significant increase in these rates again with the pandemic (Figure 2 and Figure 3). While there was a statistically significant difference (p=0.038) when the severity level ratios of DKA were examined depending on the years, this difference was found only in the severe DKA. Accordingly, the incidence of severe DKA increased in 2020 and 2021 compared to 2019 (Figure 4).
DISCUSSION
In our study, the mean annual incidence of newly diagnosed T1D in children was found to be 3.8/100.000 between 2015 and 2022 in Bursa Province. Compared to previous studies, the incidence of newly diagnosed type 1 diabetes in Bursa Province was calculated for the first time. Incidence rates of T1D have been shown to be 0.1/100.000 in China and 36.8/100.000 in Sardinia and Finland annually.3 The worldwide distribution of T1D has been published many times and varies considerably from region to region. It is emphasized that the variability might be due to differential distributions of risk genes for the disease, environmental exposures, or methodological issues.1-3,7,8,19 In Turkey, a few studies evaluate the incidence of T1D.4-6,13-15 In 2016, the first study from Turkey showed the incidence and prevalence of Type 1 diabetes in children; the incidence was 10.8/100.000.14 Moreover, 13.1/100.000 in Malatya Province (2007-2019),5 8.03/100.000 in Diyarbakır Province (2020),6 16.7/100.000 in Elazığ Province (2009-2019),15 7.2/100.000 in Southeastern of Turkey (2010-2011),13 and 8.99/100.000 in Northwest of Turkey (2013-1015)4 has been reported. The reason for the lower incidence of T1D compared to other provinces in our study was interpreted as data loss from one of the centers and proximity to Istanbul, the largest city in Turkey. The highest incidence was detected in 2017 and 2021. It was thought that the lower annual incidence values in 2019 compared to the previous and following years may have been due to more applications to the center for which data could not be obtained.
There are no differences between genders (1.06:1), similar to the literature.4,5,14-16,20,21 The median age at diagnosis was 9.23 years. When the age distribution was evaluated, it was observed that it peaked in two age groups: 10-14.9 years in males and 5-9.9 years in females. Some studies have reported that the 10-15 age group is the most common6,14,18,22; otherwise, some studies have found that the most common age group is 5-10 years old.4,5,16 Gender dominance in the peaks was only emphasized in Demirbilek et al.’s study, likely our study.13 This situation is thought to be due to the different ages at which boys and girls enter puberty.
Winter and December were the most common seasons and months for new diagnoses in all years. In many studies, the most common seasons were winter and autumn.4-6,16,20,21 It is estimated that the increase in the rate of new diagnoses in the winter and autumn seasons may be related to infectious diseases.
The incidence of newly diagnosed T1D showed a significant decrease after the pandemic declaration and lockdown in Turkey in April, May, and June 2020 compared to previous years and months, while an increase was detected after the end of the lockdown in July and August 2020. The systematic review published in 2024 examined 126 studies from 55 countries.22 The incidence of T1D during the pandemic period was higher than the pre-pandemic incidence. A study from Turkey emphasized that T1D was diagnosed more frequently during the pandemic, particularly in the summer months.18 This study highlighted that, similar to our research, the number of patients diagnosed with T1D was higher in the summer period when the number of COVID-19 cases decreased, when there were many COVID-19 cases.18 Although there is no statistical data, it is presumed that a decline in admissions is due to curfew, lockdown, or avoidance of going to hospitals and/or emergency rooms due to fear of COVID-19.
While the DKA rate at diagnosis decreased in 2019, there was a significant increase in these rates again with the pandemic. Moreover, the severe DKA rate in 2020 and 2021 increased compared to 2019. Many studies have shown a rise in DKA or DKA severity during the pandemic, similar to our study.17,18,23-25 We assume that the reason is lockdown or curfew, or even if there is no lockdown, there may be a delay in going to the hospital due to fear of COVID-19.
CONCLUSION
This study presents the first comprehensive analysis of the incidence of initially diagnosed T1D in children and adolescents in Bursa Province. The findings reveal a lower incidence rate than in other regions of Turkey, possibly due to data loss and geographical factors. The study also highlights patterns in age, gender, and seasonality of diagnoses, aligning with global trends. Notably, the COVID-19 pandemic influenced the incidence and severity of T1D presentations, with delayed diagnoses and increased DKA rates likely resulting from lockdowns and healthcare avoidance. These findings underscore the need for heightened awareness and timely intervention, particularly during global health crises, to prevent complications in pediatric T1D patients. Further research is warranted to explore regional disparities and long-term trends in T1D incidence.
Limitations of the study
The most important limitation of the study is that the data were collected from different centers, and that the data from one center could only be used in part of the study due to insufficient archiving. Additionally, we just speculated this data because there was no statistical data: The COVID-19 pandemic influenced the incidence and severity of T1D presentations, with delayed diagnoses and increased rates of DKA predicted due to lockdown and avoidance of healthcare services.
Ethical approval
This study has been approved by the Bursa Yüksek İhtisas Education and Research Hospital Clinical Research Ethics Board (approval date 19.04.2023, number 2011-KAEK-25 2023/04-21). Written informed consent was obtained from the participants.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Patterson C, Guariguata L, Dahlquist G, Soltész G, Ogle G, Silink M. Diabetes in the young - a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res Clin Pract. 2014;103:161-75. https://doi.org/10.1016/j.diabres.2013.11.005
- DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23:857-66. https://doi.org/10.1111/j.1464-5491.2006.01925.x
- Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23:1516-26. https://doi.org/10.2337/diacare.23.10.1516
- Poyrazoğlu Ş, Bundak R, Yavaş Abalı Z, et al. Incidence of type 1 diabetes in children aged below 18 years during 2013-2015 in Northwest Turkey. J Clin Res Pediatr Endocrinol. 2018;10:336-42. https://doi.org/10.4274/jcrpe.0025
- Dündar İ, Akıncı A, Çamtosun E, Kayaş L, Çiftçi N, Özçetin E. Type 1 diabetes incidence trends in a cohort of Turkish children and youth. Turk Arch Pediatr. 2023;58:539-45. https://doi.org/10.5152/TurkArchPediatr.2023.23036
- Özalkak Ş, Yıldırım R, Tunç S, et al. Revisiting the annual incidence of type 1 diabetes mellitus in children from the Southeastern Anatolian Region of Turkey: a regional report. J Clin Res Pediatr Endocrinol. 2022;14:172-178. https://doi.org/10.4274/jcrpe.galenos.2021.2021-10-7
- Patterson CC, Gyürüs E, Rosenbauer J, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55:2142-7. https://doi.org/10.1007/s00125-012-2571-8
- EURODIAB ACE Study Group. Variation and trends in incidence of childhood diabetes in Europe [Erratum in: Lancet. 2000;356(9242):1690]. Lancet. 2000;355:873-6. https://doi.org/10.1016/S0140-6736(99)07125-1
- Unsworth R, Wallace S, Oliver NS, et al. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care. 2020;43:e170-1. https://doi.org/10.2337/dc20-1551
- Marks BE, Khilnani A, Meyers A, et al. Increase in the diagnosis and severity of presentation of pediatric type 1 and type 2 diabetes during the COVID-19 pandemic. Horm Res Paediatr. 2021;94:275-84. https://doi.org/10.1159/000519797
- Kamrath C, Rosenbauer J, Tittel SR, et al. Frequency of autoantibody-negative type 1 diabetes in children, adolescents, and young adults during the first wave of the COVID-19 pandemic in Germany. Diabetes Care. 2021;44:1540-6. https://doi.org/10.2337/dc20-2791
- Tittel SR, Rosenbauer J, Kamrath C, et al. Did the COVID-19 lockdown affect the incidence of pediatric type 1 diabetes in Germany? Diabetes Care. 2020;43:e172-3. https://doi.org/10.2337/dc20-1633
- Demirbilek H, Özbek MN, Baran RT. Incidence of type 1 diabetes mellitus in Turkish children from the Southeastern Region of the country: a regional report. J Clin Res Pediatr Endocrinol. 2013;5:98-103. https://doi.org/10.4274/Jcrpe.954
- Yeşilkaya E, Cinaz P, Andıran N, et al. First report on the nationwide incidence and prevalence of Type 1 diabetes among children in Turkey. Diabet Med. 2017;34:405-10. https://doi.org/10.1111/dme.13063
- Esen I, Okdemir D. Trend of type 1 diabetes incidence in children between 2009 and 2019 in Elazig, Turkey. Pediatr Diabetes. 2020;21:460-5. https://doi.org/10.1111/pedi.12984
- Dündar İ, Akıncı A, Camtosun E, Çiftçi N, Kayas L, Nalbantoğlu Ö. Trend in initial presenting features of type 1 diabetes mellitus over a 24 year period in Turkey: a retrospective analysis of 814 cases. Turk J Pediatr. 2022;64:40-8. https://doi.org/10.24953/turkjped.2020.3580
- Jalilova A, Ata A, Demir G, et al. The effect of the SARS-CoV-2 pandemic on presentation with diabetic ketoacidosis in children with new onset type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol. 2023;15:264-7. https://doi.org/10.4274/jcrpe.galenos.2023.2022-11-4
- İzci Güllü E, Akin L, Gökler ME, Aydin M. Increased severity of presentation signs in children with newly diagnosed type 1 diabetes during the COVID-19 pandemic: a tertiary center experience. Ann Nutr Metab. 2024;80:161-70. https://doi.org/10.1159/000538322
- Patterson CC, Harjutsalo V, Rosenbauer J, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia. 2019;62:408-17. https://doi.org/10.1007/s00125-018-4763-3
- Huang A, Chen Q, Yang W, Cui Y, Wang Q, Wei H. Clinical characteristics of 683 children and adolescents, aged 0-18 years, newly diagnosed with type 1 diabetes mellitus in Henan Province: a single-center study. BMC Pediatr. 2023;23:39. https://doi.org/10.1186/s12887-023-03847-z
- Demir F, Günöz H, Saka N, et al. Epidemiologic features of type 1 diabetic patients between 0 and 18 years of age in İstanbul city. J Clin Res Pediatr Endocrinol. 2015;7:49-56. https://doi.org/10.4274/jcrpe.1694
- Hormazábal-Aguayo I, Ezzatvar Y, Huerta-Uribe N, Ramírez-Vélez R, Izquierdo M, García-Hermoso A. Incidence of type 1 diabetes mellitus in children and adolescents under 20 years of age across 55 countries from 2000 to 2022: a systematic review with meta-analysis. Diabetes Metab Res Rev. 2024;40:e3749. https://doi.org/10.1002/dmrr.3749
- Wu S, Gao Y, Guo S, et al. Characterization of newly diagnosed type 1 diabetes in children and adolescents from 2017 to 2022 in China: a single-center analysis. BMC Pediatr. 2024;24:13. https://doi.org/10.1186/s12887-023-04498-w
- Dżygało K, Nowaczyk J, Szwilling A, Kowalska A. Increased frequency of severe diabetic ketoacidosis at type 1 diabetes onset among children during COVID-19 pandemic lockdown: an observational cohort study. Pediatr Endocrinol Diabetes Metab. 2020;26:167-75. https://doi.org/10.5114/pedm.2020.101003
- Lee YL, Nasir FFWA, Selveindran NM, Zaini AA, Lim PG, Jalaludin MY. Paediatric new onset type 1 diabetes and diabetic ketoacidosis during the COVID-19 pandemic in Malaysia. Diabetes Res Clin Pract. 2023;205:110981. https://doi.org/10.1016/j.diabres.2023.110981
Copyright and license
Copyright © 2025 The author(s). This is an open-access article published by Aydın Pediatric Society under the terms of the Creative Commons Attribution License (CC BY) which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.