Abstract
Objective: Dengue is an infectious disease that burdens global public health, especially children. There are three phases of dengue infection; the last phase is the most expected, namely the recovery phase. One of the signs of this phase is platelet recovery. The platelet recovery time is still unclear because it is greatly influenced by several factors, such as immune response and lymphocyte and neutrophil activity. These factors play an important role in platelet repair and the recovery phase.
Methods: This study was a cohort prospective study. Data were obtained between January and June 2024 in Dr. Moewardi Hospital. The inclusion criteria of this study were pediatric patients diagnosed with dengue and had complete laboratory data (neutrophils, lymphocytes, and platelets) until the fifth day of fever. Data was analyzed using the Mann-Whitney test, Fisher Exact tests, and receiver Operating Characteristic (ROC) method to obtain the Area Under Curve (AUC) value, cut point, sensitivity (Sn), and specificity (Sp).
Results: This study shows absolute lymphocytes (30%; 9/30), predominance of lymphocytes (46.7%; 14/30), and Neutrophil-Lymphocyte Ratio (NLR) (63.3%; 19/30). Statistical analysis results in correlation with platelets improvement: absolute lymphocytes (AUC 77.8%, OR 5.00, Sn 66.7%, Sp 72.7%, p=0.102), predominance of lymphocytes (AUC 73.6%, OR 7.80, Sn 16.7%, Sp 66.7%, p=0.024), and NLR (AUC 78.7%, OR 10.00, Sn 91.7%, Sp 50.0%, p=0.009).
Conclusion: Supporting examination of the neutrophil-lymphocyte ratio (NLR) has been proven to be a better indicator of the recovery phase, especially in monitoring the increase in platelets in children with dengue infection compared to lymphocyte examination.
Keywords: neutrophil-lymphocyte ratio, platelet, children, dengue fever
INTRODUCTION
Dengue incidence occurs in many countries in the world, especially in tropical areas. Dengue is transmitted mainly by the Aedes aegypti mosquito.1 The spread of dengue fever from year to year is increasingly widespread, and the number of cases is also increasing. In 2017, an estimated 105 million people were infected with dengue, with 41,000 deaths and an estimated incidence of 1,371 per 100,000 population.2 The dengue virus attacks the immune system when the host’s immunity decreases. Symptoms of dengue infection that can be life-threatening are thrombocytopenia or platelet levels below normal. The normal value of platelet levels is 150,000-400,000/microliter; if platelet levels are below 150,000/microliter, intensive treatment is needed.3
Dengue infection has three main phases, namely the fever phase, the critical phase, and the recovery phase.4 The critical phase of dengue infection is the most serious because there is a significant decrease in platelets in the blood and plasma leakage, which can lead to shock and severe bleeding.5 Many studies have been conducted to find factors that can predict this phase. Simple laboratory tests such as hematological examinations are important to help improve the accuracy of the diagnosis, and the critical phase can be passed well.6 The end of the critical phase includes improvement in hematocrit levels and increased platelet counts.7
Lymphocytosis is one of the potential indicators in the healing phase of dengue cases. An increase in the number of lymphocytes is an indicator of the body’s response to a viral infection. This is because the body begins to react to the presence of dengue virus infection. However, in-depth research on this in children has not been widely used as a predictor of the healing phase.8 The change from neutrophil predominance to lymphocytes is also interesting to observe because several studies have shown that this change indicates an increase in the body’s immunity to the dengue virus. This change can be measured using the Neutrophil-Lymphocyte Ratio (NLR).9 NLR, one of the indicators of inflammation, is a predictor for predicting improvement or worsening of infection cases. A decrease in NLR is believed to be a predictor of the recovery phase in dengue infection due to an increase in lymphocyte dominance in several cases of viral infections, especially dengue.10
Establishing a diagnosis of dengue virus infection is an important thing to do in health services in hospitals. Several types of examinations can be done in establishing a diagnosis of dengue virus infection such as PCR, serology, and hematology. However, there are still many health facilities that do not have complete laboratory examinations, so simple and accurate supporting examinations are needed so that all health facilities can implement them. The Pediatrician Association has created guidelines for the diagnosis of dengue fever according to WHO, 2009. The guidelines contain simple and accurate clinical and laboratory examinations that can be performed by health services. Examination of clinical symptoms such as fever for 1-7 days, and petechiae examination, accompanied by hematology and serology supporting examinations are examinations of the accuracy of the diagnosis of dengue fever.11 Decreased platelet count is more specific as a marker of dengue virus infection compared to other viral infections.12 Serology examination of IgM and IgG antibodies also showed an increased level of accuracy in classifying primary and secondary dengue infections compared to other viral infections.13
Simple hematology tests such as lymphocytosis, NLR, and lymphocyte dominance are important laboratory tests to predict the incidence of infection in patients with various cases.14,5 Increased Absolute Lymphocyte Count (ALC), lymphocyte predominance, and changes in NLR are interesting to study as markers of the recovery phase of dengue infection associated with improvement in thrombocytopenia. This study aims to determine whether lymphocytosis, lymphocyte dominance, and NLR can be used as predictors of improvement in thrombocytopenia in children with dengue. So that it can help reduce mortality due to dengue infection.
METHODS
Context
The design of this study is a prospective cohort. The subject was collected from medical records on dengue infection patients treated at Dr. Moewardi Hospital Indonesia from February 2024 to July 2024. The type of data used is secondary data using a total sampling technique, namely, all samples found according to the time and place of study and included in the inclusion criteria.
Inclusion criteria were pediatric patients aged 0–18 years diagnosed with dengue fever and completed routine blood data from the first to the seventh day of fever.”. Determination of the diagnosis of dengue fever is based on serological examination of IgM, hematological examination of platelet levels, and physical examination of clinical symptoms. Exclusion criteria were patients with incomplete medical records and comorbid coinfection. A total of 30 pediatric patients were obtained during this study.
Data collection and measurement
The independent variable of this study is thrombocytopenia, while the dependent variables are age, sex, nutritional status, NLR, lymphocyte dominance, and lymphocytosis (day 5). Lymphocytosis (positive ≥ 1472; negative < 1472), predominance of lymphocytes in the number of leukocytes (positive: percentage of lymphocytes that exceeds the rate of other types of leukocytes; negative: percentage of lymphocytes that is lower than the percentage of other types of leukocytes), and NLR (positive < 0.8; negative ≥ 0.8) were explored as indicators of the healing phase of dengue infection. 9 Platelet levels are used as a predictor of thrombocytopenia in Dengue Hemorrhagic Fever (DHF), described using the Receiver Operating Characteristic (ROC) curve to determine the cut point and Area Under Curve (AUC) presented as the area under the ROC curve. The results were reported as sensitivity, specificity, and positive and negative predictive values.
Analysis
Bivariate data analysis uses the Chi-square test with p>0.05 to see the significance level of the relationship between the independent and dependent variables using the test. If the results do not meet the Chi-square test, then use Fisher’s exact test with p>0.05. The strength of the relationship between the variables studied is determined by calculating the Odds Ratio (OR) value.
RESULT
Baseline data
Characteristics of the subjects in 30 pediatric patients with dengue, consisting of 12 subjects in the non-increased platelet group and 18 subjects in the increased platelet group who met the inclusion and exclusion criteria (Table 1).
| *Mann-Whitney test | |||||
| Table 1. Descriptive characteristic of respondent | |||||
| Variable | Platelet | p-value | |||
| Decrease | Increase | ||||
| n | % | n | % | ||
| Age | |||||
| <1 age | 0 | 0.0 | 3 | 10.0 | 1.000* |
| 1 – 4 age | 1 | 3.3 | 2 | 6.7 | |
| 5 – 11 age | 7 | 23.3 | 6 | 20.0 | |
| 12 – 18 age | 4 | 13.3 | 7 | 23.3 | |
| Gender | |||||
| Male | 6 | 20.0 | 14 | 46.7 | 0.120* |
| Female | 6 | 20.0 | 4 | 13.3 | |
| Nutritional status | |||||
| Underweight | 2 | 6.7 | 3 | 10.0 | 0.666* |
| Normal | 10 | 33.3 | 13 | 43.3 | |
| Overweight | 0 | 0.0 | 2 | 6.7 | |
| Absolute lymphocytes | |||||
| Negative | 6 | 20.0 | 3 | 10.0 | |
| Positive | 6 | 20.0 | 15 | 50.0 | |
| Predominance of lymphocytes | |||||
| Negative | 9 | 30.0 | 5 | 16.7 | |
| Positive | 3 | 10.0 | 13 | 43.3 | |
| Neutrophil-lymphocyte ratio | |||||
| Positive | 4 | 13.3 | 15 | 50.0 | |
| Negative | 8 | 26.7 | 3 | 10.0 | |
Based on Table 1. Most respondents with platelets not increasing were aged 5 – 11 years (23.3%; 7/30), followed by those aged 12 – 18 years (13.3%; 4/30), with an average patient age of 8.8 years. The ratio of gender and absolute lymphocytes to platelets does not increase, namely 1:1. The distribution of characteristics of respondents with the most platelets not increasing was good nutritional status (33.3%; 10/30), predominance of negative lymphocytosis (30.0%; 9/30), positive NLR (26.7%; 8/30). The distribution of respondents showed that there were no differences (p-value>0.05) in terms of age, gender, and nutritional status.
Outcome measures
We observed the clinical manifestations of dengue fever patients in 30 pediatric subjects, including general symptoms of dengue cases. All subjects (100%) had a fever, and most (70%) complained of nausea (Table 2).
| Table 2. Clinical manifestations of respondents | ||
| Clinical manifestations | n | % |
| Fever | 30 | 100 |
| Headache | 12 | 40 |
| Heartburn | 4 | 13.3 |
| Petechia | 7 | 23.3 |
| Nauseous | 21 | 70 |
| Vomit | 15 | 50 |
| Diarrhea | 6 | 20 |
| Seizures | 1 | 3.3 |
| Cough | 6 | 20 |
| Have a cold | 4 | 13.3 |
| No symptoms | 17 | 56.6 |
| Average length of stay (days) | 5 (1-18) | |
The clinical manifestations of dengue patients are listed in Table 2. All patients had a fever (100%; 30/30). Headache (40.0%; 12/30), heartburn (13.3%; 4/30), petechiae (23.3%; 7/30), nausea (70.0%; 10/30), vomiting (50.0%; 15/ 30), diarrhea (20.0%; 6/30), seizures (3.3%; 1/30), cough (20.0%; 6/30), cold (13.3%; 4/30), no symptoms (56.6%; 17/30). Average length of stay 5 (1 – 18).
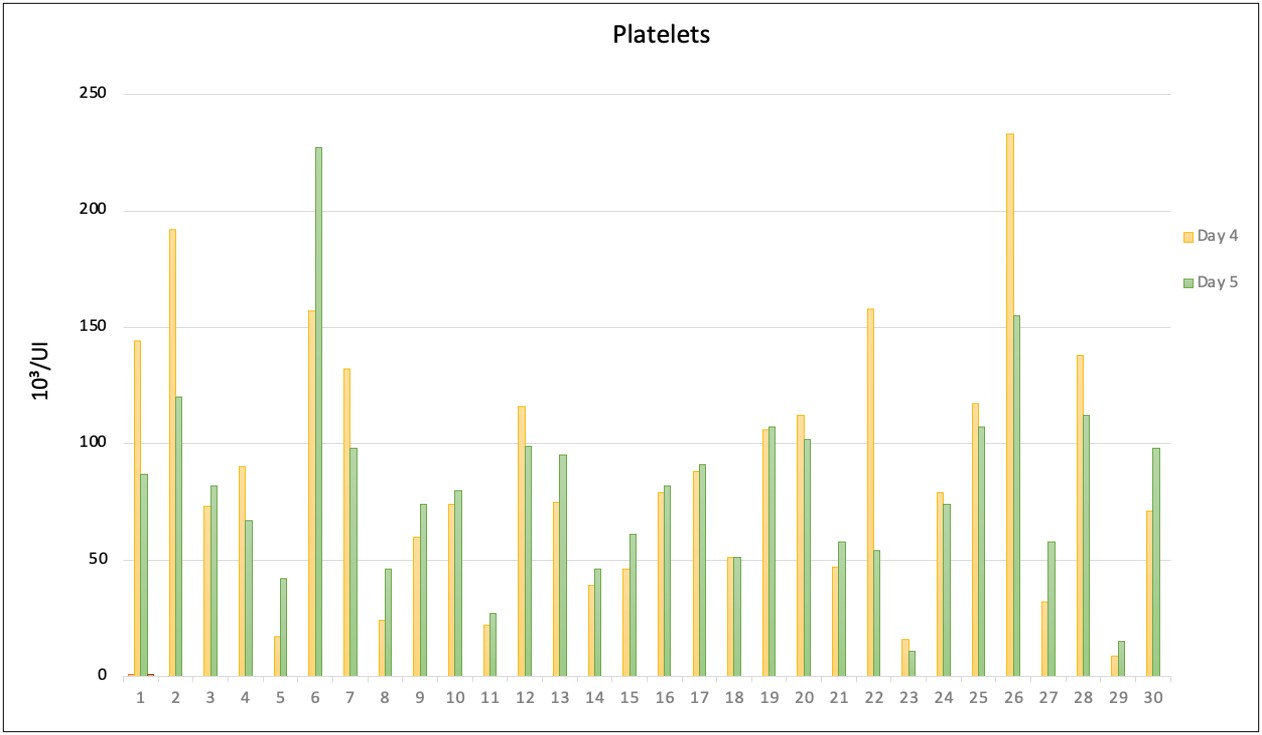
The results of observations of increased platelets based on increased lymphocytes on the fourth and fifth days in pediatric patients suffering from DHF (Figure 1). The absolute value of lymphocytes, lymphocyte dominance values, and NLR were analyzed using bivariate analysis, showing an NLR p-value of 0.009 (Table 3).
| Table 3. Bivariate variable analysis | |||
| Variable | p-value | OR | Cl 95% |
| Absolute lymphocytes | 0.102 | 5.00 | (0.933 – 26.785) |
| Predominance of lymphocytes | 0.024 | 7.80 | (1.476 – 41.214) |
| Neutrophil-lymphocyte ratio | 0.009 | 10.00 | (1.781 – 56.150) |
| *Fisher exact test | |||
Based on Table 3. The results of the bivariate analysis show that the most significant variable associated with an increase in platelets is the neutrophil-to-lymphocyte ratio (p-value = 0.009). A negative neutrophil-to-lymphocyte ratio is associated with a tenfold higher likelihood of experiencing an increase in platelets.
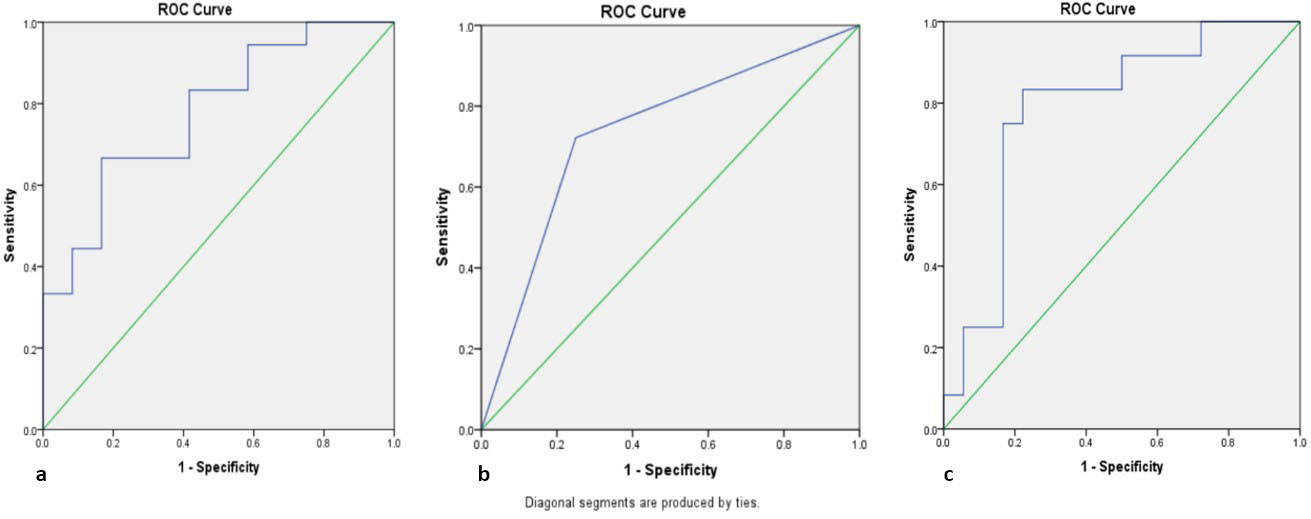
The results of further diagnostic tests comparing lymphocytosis to increased thrombosis, NLR to increased thrombosis, and lymphocyte dominance to increased thrombosis are presented with ROC curves (Figure 2, Table 4).
| Table 4. The area under curve ROC | |||||
| Variable | AUC | Cut-off | Sn | Sp | Cl 95% |
| Absolute lymphocytes | 77.8% | 2312.75 | 66.7% | 72.7% | (0.611 – 0.945) |
| Predominance of lymphocytes | 73.6% | 16.7% | 66.7% | (0.548 – 0.924) | |
| Neutrophil-lymphocyte ratio | 78.7% | 0.4748 | 91.7% | 50.0% | (0.616 – 0.958) |
| AUC: area under curve; Sn: sensitivity; Sp: specificity | |||||
Based on Table 4, a comparison of the ROC curve shows that NLR dominates in terms of sensitivity and specificity with the highest values, namely 91.7% and 50.0%, respectively. This curve also has an AUC value of 78.7% with a cut-off point of 0.4748 (Figure 2).
The results of this study show that the sensitivity in Figure 2 is 66.7%, 16.7%, and 91.7%, respectively. Based on the specificity values, the values obtained are 72.7%, 66.7%, and 50.0%. The AUC results of the three are in the sufficient category, namely 77.8%, 73.6%, and 78.7%. The ROC curve showed that NLR showed better sensitivity and AUC values than absolute lymphocytes and lymphocyte dominance.
DISCUSSION
The subjects of this study were children who had clinical symptoms of dengue fever and showed positive IgM serology results on the 5th to 7th day of fever. IgM examination is one of the important supporting examinations to determine the diagnosis of patients with dengue fever. Positive IgM indicates acute dengue infection.16
The results of the study showed that in children infected with the dengue virus in the early stages there were clinical symptoms in the form of fluctuating fever, reddish rash, petechiae, decreased platelets and accompanied by an increase or decrease in leukocytes and neutrophils. In addition, the results of this study showed several clinical symptoms that accompany dengue fever such as headache, heartburn, nausea, vomiting, diarrhea, seizures, cough, and runny nose. Headache was the most common symptom, followed by joint pain and gastrointestinal symptoms.17,18 Dengue is an acute viral infectious disease caused by the dengue virus, characterized by fever lasting 2–7 days accompanied by manifestations of bleeding, decreased platelets (thrombocytopenia), and hemoconcentration characterized by plasma leakage.19,20 In the beginning, dengue fever is characterized by leukopenia, where within 24 hours the fever will decrease and the patient will enter a critical period.21 In the initial fever phase of dengue infection, the leukocyte count can be normal or with an increase in neutrophils, followed by a decrease in the number of leukocytes and neutrophils, which reaches its lowest point at the end of the fever phase. Changes in the number (<5000 cells/mm3) and the ratio between neutrophils and lymphocytes (neutrophils<lymphocytes) are useful in predicting the critical period of plasma permeation.22,23
In this study, the sample of dengue fever respondents experienced thrombocytopenia (40%; 12/30) with an average length of hospitalization of 5 days. The risk of thrombocytopenia is directly proportional to age and is related to male gender. The male gender is more sensitive to platelet aggregation than the female.24 Thrombocytopenia in dengue virus infection causes the formation of antigen-antibody complexes when the dengue virus interacts with the body’s immune system. This complex can activate the complement system, which then causes platelet aggregation or clumping. This platelet aggregation occurs because the antigen-antibody complex attaches to the platelet membrane, which triggers the release of adenosine diphosphate (ADP), which causes platelets to stick together. This causes platelets to be destroyed by the Reticuloendothelial System (RES), which results in thrombocytopenia.25
The results of this study indicate that there is an increase in the number of platelets marked by lymphocyte predominance, which is a sign of clinical improvement in respondents. The results of the bivariate test support that lymphocyte dominance is related to an increase in platelets. Lymphocytes peaked in the convalescent phase (days 5 to 9 after fever), with the highest number recorded on day 7.26 The increase in lymphocytes in the convalescent phase is by the theory of pathogenesis involving lymphocytes, especially cytotoxic T cells in the convalescent phase, where the respondent’s condition improves.27
The absolute number of lymphocytes began to increase since day 4, but in this study there was no significant correlation between the absolute number of lymphocytes and the increase in platelets. Absolute lymphocytes increased along with the development of fever and peaked on day 7 of fever, which is the convalescent phase (days 5 to 9 of fever). The increase in absolute lymphocytes is related to the body’s reaction to the increase in the number of platelets and clinical improvement. Previous studies have revealed that although there is an increase in the absolute number of lymphocytes and the number of leukocytes in the critical phase, the increase in the absolute number of lymphocytes is more significant than the number of leukocytes. This is what causes the increase in the relative proportion of lymphocytes in the critical phase.28,29
Supporting data from simple laboratory tests with hematology tests is very helpful for fellow doctors to determine a diagnosis quickly. In cases of children with dengue fever, decreased platelets are one of the important things in establishing a diagnosis of dengue fever quickly. However, decreased or increased platelets are not only related to the occurrence of dengue infection, but several other factors can influence such as drug side effects and others. Additional laboratory tests such as NLR can help fellow doctors determine the diagnosis of dengue infection and help observe recovery from dengue fever. In this study, NRL was shown to be significantly correlated with increased platelets in children with dengue fever. Previous studies have shown a significant relationship between NLR and the severity of dengue. There is neutropenia and lymphocytosis at the beginning of fever, which can be used as predictors of infection diagnosis in the first few days of fever.30 Neutropenia and lymphocytosis can indirectly describe the neutrophil-lymphocyte ratio value; namely, if the neutrophil value is lower, the neutrophil-lymphocyte ratio will also be lower. Conversely, the higher the lymphocyte value, the lower the neutrophil-lymphocyte ratio. Neutropenia and lymphocytes play a central role in the response to dengue infection.31 Neurophils, as a type of white blood cell, move to the area of infection to fight the causative agent of the disease. After circulating in the blood for 7–10 hours, neutrophils move to the affected infection site to participate in the body’s immune response.32 During dengue virus infection, the bone marrow is suppressed, either directly caused by the virus itself or indirectly through the production of proinflammatory cytokines that inhibit bone marrow activity.33
Our study still has some limitations, such as the number of dengue cases treated in the hospital is still small and the treatment time is limited. However, this study is the first study to examine the role of lymphocytosis, lymphocyte dominance, and NLR as predictors of increased platelets and indicators of recovery in pediatric dengue fever. The need for other simple supporting examinations that can be indicators of the recovery phase in dengue diabetes is very much needed by medical colleagues in daily practice, especially in pediatrics. Our further research will focus on tighter control of confounding variables to achieve the best results in dengue infection.
CONCLUSION
On day four, fever, lymphocytosis, lymphocyte predominance, and NLR can serve as predictors of the convalescent phase, especially in monitoring improvement in platelets. NLR has proven to be a better predictor of the convalescent phase, especially in monitoring improvement in platelets in children with dengue infection.
Acknowledgment
We thank the patient and family for allowing us to participate in conducting ancillary examinations to diagnose dengue infection.
Ethical approval
This study has been approved by the Ethics Committee of Dr. Moewardi General Hospital, Indonesia (approval date 15.02.2024, number 422/II/HREC/2024). Written informed consent was obtained from the participant’s parent.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB. Dengue infection. Nat Rev Dis Primers. 2016;2:16055. https://doi.org/10.1038/nrdp.2016.55
- Zeng Z, Zhan J, Chen L, Chen H, Cheng S. Global, regional, and national dengue burden from 1990 to 2017: A systematic analysis based on the global burden of disease study 2017. EClinicalMedicine. 2021;32:100712. https://doi.org/10.1016/j.eclinm.2020.100712
- Pinheiro MBM, Rozini SV, Quirino-Teixeira AC, et al. Dengue induces iNOS expression and nitric oxide synthesis in platelets through IL-1R. Front Immunol. 2022;13:1029213. https://doi.org/10.3389/fimmu.2022.1029213
- Wickramasinghe W, Alvitigala BY, Perera T, et al. Rotational thromboelastometry in critical phase of dengue infection: association with bleeding. Res Pract Thromb Haemost. 2022;6:e12704. https://doi.org/10.1002/rth2.12704
- Tayal A, Kabra SK, Lodha R. Management of dengue: an updated review. Indian J Pediatr. 2023;90:168-77. https://doi.org/10.1007/s12098-022-04394-8
- Thach TQ, Eisa HG, Hmeda AB, et al. Predictive markers for the early prognosis of dengue severity: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2021;15:e0009808. https://doi.org/10.1371/journal.pntd.0009808
- Kularatne SA, Dalugama C. Dengue infection: global importance, immunopathology and management. Clin Med (Lond). 2022;22:9-13. https://doi.org/10.7861/clinmed.2021-0791
- Ananda Rao A, U RR, Gosavi S, Menon S. Dengue fever: prognostic insights from a complete blood count. Cureus. 2020;12:e11594. https://doi.org/10.7759/cureus.11594
- Chaloemwong J, Tantiworawit A, Rattanathammethee T, et al. Useful clinical features and hematological parameters for the diagnosis of dengue infection in patients with acute febrile illness: a retrospective study. BMC Hematol. 2018;18:20. https://doi.org/10.1186/s12878-018-0116-1
- Sadgir A, Durge K, Masavkar S. Neutrophil-lymphocyte ratio as a prognostic indicator in dengue fever patients at tertiary care hospital in Northwest-Maharashtra. Int J Adv Res. 2023;11:1198-202. https://doi.org/10.21474/IJAR01/16137
- Wang WH, Urbina AN, Chang MR, et al. Dengue hemorrhagic fever - a systemic literature review of current perspectives on pathogenesis, prevention and control. J Microbiol Immunol Infect. 2020;53:963-78. https://doi.org/10.1016/j.jmii.2020.03.007
- Kulkarni RD, Patil SS, Ajantha GS, et al. Association of platelet count and serological markers of dengue infection-importance of NS1 antigen. Indian J Med Microbiol. 2011;29:359-62. https://doi.org/10.4103/0255-0857.90159
- Blacksell SD, Jarman RG, Bailey MS, et al. Evaluation of six commercial point-of-care tests for diagnosis of acute dengue infections: the need for combining NS1 antigen and IgM/IgG antibody detection to achieve acceptable levels of accuracy. Clin Vaccine Immunol. 2011;18:2095-101. https://doi.org/10.1128/CVI.05285-11
- Russell CD, Parajuli A, Gale HJ, et al. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: a systematic review and meta-analysis. J Infect. 2019;78:339-48. https://doi.org/10.1016/j.jinf.2019.02.006
- Kalinichenko S, Komkov D, Mazurov D. HIV-1 and HTLV-1 transmission modes: mechanisms and importance for virus spread. Viruses. 2022;14:152. https://doi.org/10.3390/v14010152
- Valdivia-Conroy B, Vasquez-Calderón JM, Silva-Caso W, et al. Diagnostic performance of the rapid test for the detection of NS1 antigen and IgM and IgG anti-antibodies against dengue virus. Rev Peru Med Exp Salud Publica. 2022;39:434-41. https://doi.org/10.17843/rpmesp.2022.394.11471
- Gregory CJ, Santiago LM, Argüello DF, Hunsperger E, Tomashek KM. Clinical and laboratory features that differentiate dengue from other febrile illnesses in an endemic area-Puerto Rico, 2007-2008. Am J Trop Med Hyg. 2010;82:922-9. https://doi.org/10.4269/ajtmh.2010.09-0552
- Biswas HH, Ortega O, Gordon A, et al. Early clinical features of dengue virus infection in nicaraguan children: a longitudinal analysis. PLoS Negl Trop Dis. 2012;6:e1562. https://doi.org/10.1371/journal.pntd.0001562
- Teo D, Ng LC, Lam S. Is dengue a threat to the blood supply? Transfus Med. 2009;19:66-77. https://doi.org/10.1111/j.1365-3148.2009.00916.x
- Huy BV, Toàn NV. Prognostic indicators associated with progresses of severe dengue. PLoS One. 2022;17:e0262096. https://doi.org/10.1371/journal.pone.0262096
- Sabchareon A, Sirivichayakul C, Limkittikul K, et al. Dengue infection in children in Ratchaburi, Thailand: a cohort study. I. Epidemiology of symptomatic acute dengue infection in children, 2006-2009. PLoS Negl Trop Dis. 2012;6:e1732. https://doi.org/10.1371/journal.pntd.0001732
- Kotepui M, PhunPhuech B, Phiwklam N, Uthaisar K. Differentiating between dengue fever and malaria using hematological parameters in endemic areas of Thailand. Infect Dis Poverty. 2017;6:27. https://doi.org/10.1186/s40249-017-0238-x
- Ye G, Xu Z, Yang M, et al. Clinical features and transmission risk analysis of dengue virus infections in Shenzhen, During 2014-2019. Comput Struct Biotechnol J. 2023;21:3728-35. https://doi.org/10.1016/j.csbj.2023.07.001
- Tsheten T, Clements ACA, Gray DJ, Adhikary RK, Furuya-Kanamori L, Wangdi K. Clinical predictors of severe dengue: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10:123. https://doi.org/10.1186/s40249-021-00908-2
- Roy S. Thrombopoietin receptor agonists: can these be the future answer to the deadly thrombocytopenia in dengue fever? Cureus. 2019;11:1-12. https://doi.org/10.7759/cureus.4361
- Riswari SF, Tunjungputri RN, Kullaya V, et al. Desialylation of platelets induced by Von Willebrand Factor is a novel mechanism of platelet clearance in dengue. PLoS Pathog. 2019;15:e1007500. https://doi.org/10.1371/journal.ppat.1007500
- Ji X, Zhang L, Peng J, Hou M. T cell immune abnormalities in immune thrombocytopenia. J Hematol Oncol. 2014;7:72. https://doi.org/10.1186/s13045-014-0072-6
- Rashmi MV, Hamsaveena. Haematological and biochemical markers as predictors of dengue infection. Malays J Pathol. 2015;37:247-51.
- Godoy-Lozano EE, Téllez-Sosa J, Sánchez-González G, et al. Lower IgG somatic hypermutation rates during acute dengue virus infection is compatible with a germinal center-independent B cell response. Genome Med. 2016;8:23. https://doi.org/10.1186/s13073-016-0276-1
- Tran HTD, Schindler C, Pham TTT, et al. Simple clinical and laboratory predictors to improve empirical treatment strategies in areas of high scrub typhus and dengue endemicity, central Vietnam. PLoS Negl Trop Dis. 2022;16:e0010281. https://doi.org/10.1371/journal.pntd.0010281
- Rosso F, Parra-Lara LG, Agudelo-Rojas OL, Martinez-Ruiz DM. Differentiating dengue from COVID-19: comparison of cases in Colombia. Am J Trop Med Hyg. 2021;105:745-50. https://doi.org/10.4269/ajtmh.20-0912
- Recker M, Fleischmann WA, Nghia TH, et al. Markers of prolonged hospitalisation in severe dengue. PLoS Negl Trop Dis. 2024;18:e0011922. https://doi.org/10.1371/journal.pntd.0011922
- Ab-Rahman HA, Wong PF, Rahim H, et al. Dengue death with evidence of hemophagocytic syndrome and dengue virus infection in the bone marrow. Springerplus. 2015;4:665. https://doi.org/10.1186/s40064-015-1463-z
Copyright and license
Copyright © 2025 The author(s). This is an open-access article published by Aydın Pediatric Society under the terms of the Creative Commons Attribution License (CC BY) which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.