Abstract
Objective: The primary objective of our study was to investigate the growth patterns of extremely low gestational age newborns (ELGAN) in the Neonatal Intensive Care Unit (NICU), assess the prevalence of Extrauterine Growth Restriction (EUGR) among them, and identify factors influencing its development. Additionally, the study aimed to evaluate the consistency between cross-sectional and longitudinal EUGR in ELGANs and assess catch-up growth at corrected 24 months.
Method: Growth patterns of ELGANs and additional clinical data were retrospectively collected from January 2021 to January 2022 at a single tertiary NICU. EUGR was defined using two methods: cross-sectional EUGR and longitudinal EUGR. Infants were classified into two groups—EUGR and non-EUGR—based on whether their weight z-score was below -1.28 at the time of evaluation (either at a corrected gestational age (CGA) of 36 weeks or at discharge, whichever occurred first) or if the z-score decline (ΔZ score) exceeded 1 standard deviation (SD) between birth and the time of evaluation. According to WHO Child Growth Standards, catch-up growth was assessed at the age of two.
Results: The study included 66 ELGANs. The incidence of EUGR was 51.5% (34 out of 66) based on the cross-sectional definition, increasing to 74.2% (49 out of 66) under the longitudinal definition. Using the criterion of a ΔZ weight < –1, the EUGR group took longer to achieve total enteral nutrition and required more days of total parenteral nutrition than the non-EUGR group. Additionally, the average weight growth velocity (GV) was significantly lower in the EUGR group. Late-onset sepsis (LOS), cumulative antibiotic exposure, and feeding intolerance (FI) were significantly more prevalent in the EUGR group. Among ELGANs discharged with EUGR (based on the longitudinal definition), 53% achieved catch-up growth in weight by one year of age and 77% by two years.
Conclusion: The present study highlights the importance of LOS as an independent risk factor for developing EUGR and underscores the need for interventions to reduce its incidence. Additionally, enhanced enteral nutrition support and strategies to promote higher growth velocity may effectively reduce the incidence of extrauterine growth restriction in ELGANs. Approximately 25% of ELGAN infants are expected to remain underweight by the age of two years, while the majority achieve normalization of head circumference.
Keywords: extremely low gestational age newborns (ELGAN), extrauterine growth Restriction (EUGR), postnatal growth, catch-up growth
INTRODUCTION
Historically, the target growth of preterm infants has been to replicate intrauterine growth patterns, as established by the American Academy of Pediatrics (AAP) in 1977.1 However, consensus on the optimal growth trajectory for preterm infants and the methodologies for monitoring growth in the neonatal intensive care unit (NICU) remains elusive. The most commonly used growth charts are the cross-sectional charts derived from in-utero growth data. Whether preterm infants should be expected to grow at the same rate as their in-utero counterparts is still debated.2 Extrauterine Growth Restriction (EUGR) refers to inadequate growth in preterm infants in the NICU.3 EUGR significantly affects multiple aspects of a premature infant’s health and development.4 Research has shown that growth retardation is associated with adverse neurodevelopmental outcomes.3,5 These findings underscore the importance of monitoring and intervening in the postnatal growth of premature infants to reduce the incidence of EUGR and promote their neurodevelopment and growth. EUGR is classified into two types: Cross-sectional EUGR could be defined as below the 10th percentile or age specific weight Z-score below the −1.28 at a given time. Longitudinal EUGR could be described as an age-specific weight Z-score fall of more than 1 standard deviation (SD) between birth and the given time.6 The Z-score measures the number of standard deviations an infant’s weight and height are from the median, or the 50th percentile, of the reference growth charts for infants of the same age and sex. The World Health Organization (WHO) defines Small for Gestational Age (SGA) as a newborn with a birth weight below the 10th percentile for infants of the same sex and gestational age. EUGR in SGA infants may represent a continuation of intrauterine growth retardation rather than “true EUGR.” Hence, it is advised to assess EUGR in SGA infants as a distinct category.7 Extremely low gestational age newborns (ELGANs) or extremely preterm infants (EPIs) are born before 28 completed weeks of gestation (up to and including 27 weeks and 6 days of gestation).8 ELGANs are at high risk of unsuccessful postnatal adaptation, including challenges with initiating enteral nutrition, achieving full enteral nutrition, and attaining optimal postnatal growth. While the longitudinal definition of EUGR is generally considered more predictive of long-term outcomes in preterm infants, data on ELGANs is scarce. This study aimed to compare the cross-sectional and longitudinal definitions of EUGR in determining the true prevalence of EUGR in ELGAN infants, the influencing factors, and the growth status at 24 months.
OBJECTIVE AND METHODS
The study included infants born at Tinaztepe University Galen Hospital or transferred there within the first day of life between January 2021 and January 2022. Only ELGANs (infants born before 28 weeks of gestation) were included. Infants with SGA, major birth defects, or congenital anomalies were excluded. Birth weight and weight at the time of evaluation (either at a corrected gestational age (CGA) of 36 weeks or at discharge, whichever occurred first) were converted into age-specific and sex-specific Z-scores using the 2013 Fenton dataset.9 SGA was defined as an age-specific birth weight Z-score below −1.28, according to the Fenton growth chart.9 Cross-sectional EUGR is defined as an age-specific weight Z-score below -1.28 at the time of evaluation, either at a CGA of 36 weeks or at discharge, whichever occurs first. Longitudinal EUGR is defined as a decline in the Z-score (ΔZ score) of more than one SD from birth to the specified time.6
Perinatal data, maternal and pregnancy complications, growth and nutritional status during hospitalization, treatment conditions, major complications, and other clinical data of ELGANs were retrospectively collected from medical records. Data on growth and nutritional status during hospitalization included maximum weight loss, age at birth, weight recovery, the average weight gain velocity (GV), start time of enteral feeding (excluding colostrum oral care), breast milk volume after the addition of human milk fortifier (HMF), the age of reaching total enteral nutrition and age at reaching the target oral calorie intake (110 kcal/kg/day), the duration of parenteral nutrition (PN). These data were collected from medical records. The average weight GV was expressed as g/kg/day and calculated using Patel’s method with the following equation: Growth velocity = 1000 × Ln (Wt2/Wt1)/ (D2 − D1) where Wt₁ and Wt₂ represent the infant’s weight measured on days (D) 1 and D2, respectively.10 The age at which total enteral nutrition was achieved was defined as the number of days required to reach a target oral calorie intake of 110 kcal/kg/day.
Data on invasive mechanical ventilation duration, total oxygen use duration, cumulative antibiotic use, postnatal steroid treatment, and hemodynamically significant patent ductus arteriosus (hsPDA) were collected from medical records. Additionally, information on early-onset sepsis (EOS), late-onset sepsis (LOS), feeding intolerance (FI), neonatal necrotizing enterocolitis (NEC) ≥ stage 2 (according to Bell’s classification11), bronchopulmonary dysplasia (BPD), grade III-IV intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), and retinopathy of prematurity (ROP) requiring intervention based on established diagnostic criteria12 was also obtained. Bronchopulmonary dysplasia (BPD) was defined as a continuous oxygen requirement for the first 28 days and a need for oxygen at 36 weeks postmenstrual age.13 The diagnostic criteria for EOS and LOS were established through expert consensus and have been used to diagnose and treat neonatal sepsis.14 According to the local NICU protocol, feeding intolerance was defined as failure of the feeding plan, characterized by gastric residue exceeding 50% of the previous feeding amount or gastric residue containing bile, accompanied by vomiting and/or abdominal distension. According to WHO Child Growth Standards, catch-up growth was evaluated at the age of two years. Low weight for age (underweight), low height for age (stunting), and reduced head circumference for age were diagnosed when Z-scores fell below -2 standard deviations. The study was approved by the clinical research ethics committee of Izmir Tinaztepe University with decision no 2024/62. Ethical principles were adhered to, and the research was conducted in accordance with the Declaration of Helsinki.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY, USA). Normally distributed measurement data were expressed as mean ± SD, and comparisons between groups were made using independent-sample t-tests. Non-normally distributed quantitative data were reported as median and interquartile ranges (IQRs), with group comparisons performed using the Mann–Whitney U test. Categorical variables were analyzed using the Chi-square test, with Fisher’s exact test applied when necessary. Univariate analysis was conducted to identify potential factors influencing clinical outcomes. A p-value < 0.05 was considered statistically significant. All differences among and between groups were considered to be statistically significant at P < 0.05
RESULT
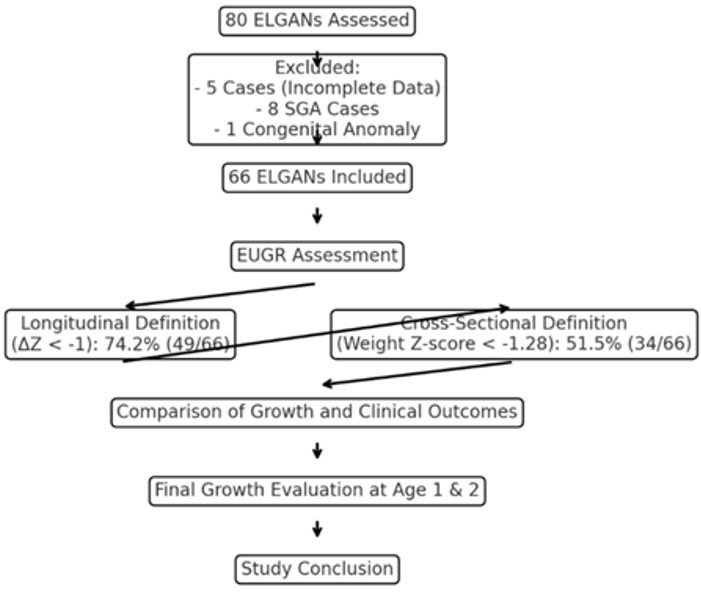
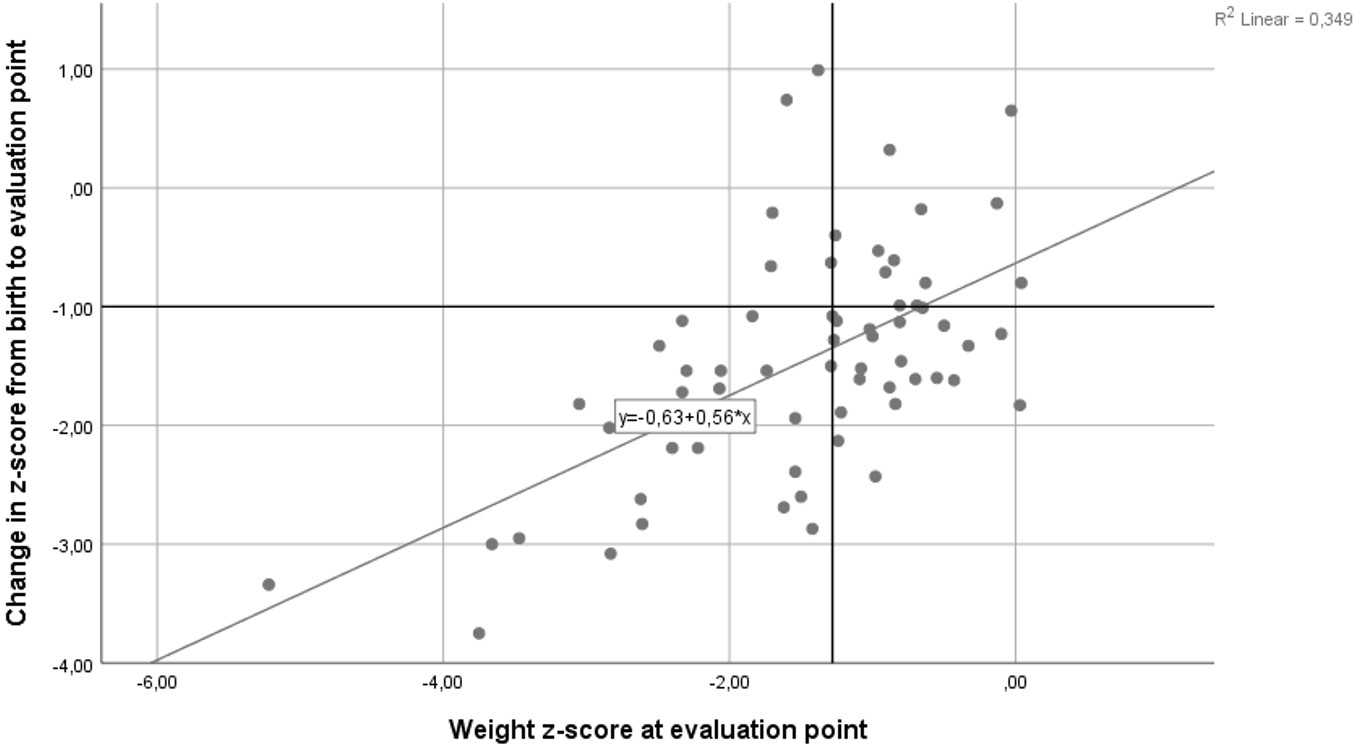
Throughout the study period, data were collected on 80 ELGANs. Five cases were excluded due to incomplete information, and an additional nine cases were excluded (eight due to SGA and one due to congenital anomalies). Ultimately, 66 ELGANs were included and assessed in the study (Figure 1). The average birth weight of the infants included in the study was 807 ± 206 grams, and the average gestational age was 25.4 ± 1.2 weeks. The incidence of EUGR among ELGANs was 74.2% (49 out of 66 cases) when assessed using the longitudinal definition based on infant weight at 36 weeks of CGA or at discharge. In contrast, when assessed using the cross-sectional definition, the incidence of EUGR was 51.5% (34 out of 66 cases). Figure 2 presents a scatterplot of the weight-for-age Z-score plotted against the change in weight-for-age Z-score (ΔZ). Using the ΔZ < –1 criterion, birth weight and weight Z-score at birth did not differ significantly between the EUGR and non-EUGR groups. Similarly, birth length, birth length Z-score, birth head circumference, and birth head circumference Z-score did not differ significantly between the EUGR and non-EUGR groups. Additionally, factors such as gestational age, the incidence of female infants, 5-minute Apgar scores, and other perinatal and neonatal variables—including pregnancy-induced hypertension, gestational diabetes, mode of delivery, multiple births, and antenatal steroid administration—showed no significant differences between the EUGR and non-EUGR groups (P > 0.05), as presented in Table 1.
| EUGR: extrauterine growth restriction | |||
| Table 1. Comparison of general perinatal and natal characteristics between EUGR and non-EUGR groups | |||
| Variable | Non-EUGR n: 27 | EUGR n: 39 | p |
| Female [n (%)] | 16 (59.2%) | 20 (51.2%) | 0.52 |
| Gestational weeks (mean±SD) | 25.3±1.27 | 25.5±1.31 | 0.55 |
| Birth weight, g (mean±SD) | 761±187 | 838±215 | 0.12 |
| Weight z score at birth (mean±SD) | 0.19±0.72 | 0.24±0.87 | 0.13 |
| Birth length, cm (mean±SD) | 32.69±3.02 | 33.48±2.72 | 0.27 |
| Length z score at birth (mean±SD) | 0.09±0.94 | 0.27±0.90 | 0.4 |
| Birth head circumference, cm (mean±SD) | 23.75±2.12 | 24.06±2.3 | 0.58 |
| Head circumference z score at birth (mean±SD) | 0.46±0.98 | 0.51±1.09 | 0.84 |
| Cesarean section [n (%)] | 22 (81.4%) | 31 (79.4%) | 0.36 |
| Multiple births [n (%)] | 2 (7.4%) | 5 (12.8%) | 0.12 |
| Antenatal steroid treatment [n (%)] | 15 (55.5%) | 23 (58.9%) | 0.62 |
| Gestational hypertension [n (%)] | 2 (7.4%) | 2 (5.1%) | 0.53 |
| Gestational diabetes [n (%)] | 1 (3.7%) | 3 (7.6%) | 0.11 |
| Gestational age at discharge (weeks, mean ± SD) | 39.18±1.86 | 39.17±2.07 | 0.77 |
Following the criterion of ΔZ of weight < –1, a comparison between the EUGR and non-EUGR groups showed no significant differences in maximum physiological weight loss, the age at which birth weight was regained, or the initiation of enteral feeding. However, the average weight GV was significantly lower in the EUGR group (19.5±3.3 vs. 22.6±3). Additionally, the age at which total enteral nutrition was achieved, the number of days required to reach the target oral calorie intake (110 kcal/kg/day), and the duration of parenteral nutrition were all significantly greater in the EUGR group compared to the non-EUGR group (P < 0.05), as presented in Table 2. Following the criterion of ΔZ of weight < –1, the LOS, cumulative duration of antibiotics uses, and incidences of FI in the EUGR group were significantly higher than in the non-EUGR group (P < 0.05). However, the incidences of complications such as RDS, EOS, HsPDA, IVH grades 3-4, BPD, NEC stage 2 or higher, PVL, and ROP requiring intervention did not differ significantly between the groups (P > 0.05) as shown in Table 3.
| Table 2. Comparison of the nutritional status of ELGANs between the EUGR and non-EUGR groups during hospitalization | |||
| Variable | Non-EUGR n:27 | EUGR n:39 | p |
| Maximum physiological weight loss, % | 10.4±5.1 | 11.5±3.6 | 0.3 |
| Age at birth weight recovery (days) | 11.2±4.9 | 13.4±5.6 | 0.12 |
| Average weight gain velocity (GV) (g/kg/day) | 22.6±3 | 19.5±3.3 | 0.01* |
| Initiation of enteral feeding (days) | 4±2.4 | 4.5±3 | 0.5 |
| Volume of milk fortified with HMF (ml/kg) | 87.1±13.9 | 81.6±12.9 | 0.11 |
| Time to reach target oral calorie intake (110 kcal/kg/day) (days) | 48.2±15.4 | 55.7±17.6 | 0.01* |
| Duration of parenteral nutrition (days) | 39.4±13.8 | 45.4±18.5 | 0.03* |
|
ELGAN: extremely low gestational age newborns; EUGR: extrauterine growth restriction; HMF: human milk fortifier *p<0.05 |
|||
|
ELGAN: extremely low gestational age newborns; EUGR: extrauterine growth restriction; PDA: patent ductus arteriosus *p<0.05 |
|||
| Table 3. Comparison of the main treatments and complications related to hospitalization of ELGANs between the EUGR and non-EUGR groups | |||
| Variable | Non-EUGR n:27 | EUGR n:39 | p |
| Invasive ventilation duration (days) | 42.9±33.6 | 29.8±30.4 | 0.11 |
| Total oxygen therapy duration (days) | 64.1±35.2 | 57.8±36.2 | 0.48 |
| Cumulative duration of antibiotic use (days) | 14.8±6.5 | 20.8±8.3 | 0.04* |
| Postnatal steroid treatment [n (%)] | 15 (55.5%) | 22 (56.4%) | 0.78 |
| Hemodynamically significant PDA (hsPDA) [n (%)] | 21 (77.7%) | 28 (71.7%) | 0.58 |
| Early-onset sepsis (EOS) [n (%)] | 5 (18.5%) | 11 (28.2%) | 0.36 |
| Feeding intolerance (FI) [n (%)] | 15(55.5%) | 32(82%) | 0.03* |
| Late-onset sepsis (LOS) [n (%)] | 14 (51.8%) | 34 (87.2%) | 0.02* |
| Necrotizing enterocolitis (NEC ≥ stage 2) [n (%)] | 2 (7.4%) | 3 (7.7%) | 0.96 |
| Bronchopulmonary dysplasia (BPD) [n (%)] | 12 (44.4%) | 10 (25.6%) | 0.11 |
| Periventricular leukomalacia (PVL) [n (%)] | 2 (7.4%) | 3 (7.7%) | 0.96 |
| Intraventricular hemorrhage (IVH, grade 3-4) [n (%)] | 1 (3.7%) | 4 (10.3%) | 0.32 |
| Retinopathy of prematurity (ROP, requiring intervention) [n (%)] | 2 (7.4%) | 3 (7.7%) | 0.96 |
The discharge weight of the EUGR group was significantly lower than that of the non-EUGR group (2536 ± 287 g vs. 2880 ± 469 g, p = 0.01). Upon evaluating ELGANs for growth at the age of one, it was found that 47% of infants were underweight, 29% experienced stunting, and 38% had a reduced head circumference for their age. By the age of two, these rates had decreased to 23% for underweight, 15% for stunting, and 6% for reduced head circumference.
DISCUSSION
The Fenton growth curves, revised in 2013 and based on data from a large cohort of preterm infants, are widely used to evaluate both intrauterine and extrauterine growth in this population.9 Our study identified a significant discrepancy in EUGR assessment depending on whether it was based on the p-value or the ΔZ-score on the growth curve, either at the adjusted 36th week or at discharge, within the same population. Specifically, the incidence of EUGR among ELGANs was 74.2% when defined by a ΔZ-score of less than –1, compared to 51.5% when defined by a discharge weight p-value of less than 10%. This discrepancy of 22.7% highlights the impact of varying EUGR definitions within the same population. Although various studies have reported a higher incidence of EUGR when using the cross-sectional definition based on a discharge weight Z-score < –1.28 (equivalent to a p-value < 10th percentile) compared to the longitudinal definition, longitudinal assessment is considered a more accurate reflection of neonates’ true growth trajectories. 6,15 Since the p-value evaluation method is based on the horizontal analysis of group data, whereas the ΔZ score is derived from the analysis of individual data.16 Simon et al. proposed that the change in Z scores from birth weight to weight at discharge (ΔZ score) be incorporated into the longitudinal definition for assessing EUGR in premature infants to more accurately reflect their postnatal growth status.17 De Rose et al. proposed that a longitudinal definition for EUGR is more effective than a cross-sectional definition in predicting adverse neurodevelopmental outcomes at a two-year follow-up.18 Therefore, the definition based on the ΔZ score is thought to be more effective in predicting the long-term outcomes for preterm infants. In our research, we employed the longitudinal definition of EUGR, which is based on the ΔZ score, to compare the EUGR and non-EUGR groups in ELGANs. Numerous studies have indicated that higher birth weights and male gender may act as protective factors against EUGR.19-21 Nevertheless, our research revealed no significant differences in birth weight and gender between the EUGR and non-EUGR groups. Postnatal nutritional status is closely linked to the incidence of EUGR. Current guidelines indicate that premature infants need careful monitoring of their growth and the proper and consistent provision of nutrients. This includes supplementation with breast milk, provided both parenterally and enterally, particularly in the first weeks of life.22 In our study, the univariate analysis revealed that the non-EUGR group exhibited a higher average weight GV (p=0.01), achieved the target oral calorie intake earlier (p=0.01), and had a shorter duration of parenteral nutrition (p=0.03) compared to the EUGR group. Oral calorie intake reaching 110 kcal/kg has been shown to be protective against EUGR in ELGANs, and this is associated with shorter TPN duration and higher GV. Although the HMF initiation time did not differ between the groups in our study, HMF is quite important in reaching the target oral calorie target in preterm infants. Studies have also found that infants experiencing EUGR received fewer calories and less protein than recommended during the transition from parenteral to enteral feeding.23 The findings indicate that increased focus on enteral nutrition support for ELGANs is warranted. Such factors are crucial in diminishing the occurrence of EUGR. European Milk Bank Association (EMBA) recommends using individualized fortification to optimize nutrient intake.24 In this study, all ELGANs utilized the individualized fortification method based on blood urea nitrogen (BUN) levels as the standard protocol for HMF. Our study indicated that the occurrence of LOS, the overall length of antibiotic therapy, and instances of feeding intolerance were more prevalent in infants with EUGR compared to their counterparts without this condition. The increased incidence of LOS in ELGANs with EUGR may be linked to feeding intolerance, which is often a consequence of extended antibiotic treatment and significant disruption of intestinal microbiota. Major morbidities linked to prematurity, including PDA, BPD, NEC, the requirement for assisted ventilation, exposure to postnatal steroids, and severe brain lesions, substantially impact the incidence of growth restriction and increase the risk of developing extrauterine growth restriction.25,26 Greenbury et al.’s extensive study demonstrated significant growth restriction in extremely premature infants who suffer from these major morbidities.27 Our study revealed no significant differences in major morbidities between the EUGR and non-EUGR groups, except LOS. These morbidities might simply indicate the severity of illness; sick infants tend to be fed less than their healthier counterparts, face higher metabolic demands, and often have unmet nutritional needs, leading to malnutrition and stunted growth. In 2021, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (EPSGHAN) emphasized that to catch up with growth in preterm infants with major morbidity, ongoing energy and protein needs must be met during this period. It has been recommended that critically ill premature newborns replace nutritional and energy deficiencies by increasing calories to 160 kcal/kg/day, protein to 4.5 g/kg/day, glucose to 12.5 g/kg/day, and fat to 8 g/kg/day during the recovery phase.28 There is limited information on the amount of energy and nutrients that should be provided to meet the increased metabolic requirements due to major morbidities in a specific group, including ELGANs. Given the increasing rates of extremely premature survivors, a universal definition of EUGR and guidelines on neonatal feeding are essential.
The limitations of this study include a single-center design, a small sample size, variability in results due to different EUGR definitions, the absence of long-term neurodevelopmental data, and the lack of detailed evaluation of nutritional and environmental factors. However, we believe it will contribute to the literature by presenting data on a subject for which there is limited information, such as extrauterine growth patterns and catch-up growth in the first 2 years of life in extremely preterm infants. The present study highlights the importance of LOS as an independent risk factor for the development of EUGR and the need for interventions aimed at reducing its incidence.
Ethical approval
This study has been approved by the Tınaztepe University Ethics Committee (approval date 10.10.2024, number 2024-62). Written informed consent was obtained from the participants.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- American Academy of Pediatrics, Committee on Nutrition. Nutritional needs of low-birth-weight infants. Pediatrics. 1977;60:519-30. https://doi.org/10.1542/peds.60.4.519
- El Rafei R, Jarreau PH, Norman M, et al. Variation in very preterm extrauterine growth in a European multicountry cohort. Arch Dis Child Fetal Neonatal Ed. 2021;106:316-23. https://doi.org/10.1136/archdischild-2020-319946
- González-López C, Solís-Sánchez G, Lareu-Vidal S, et al. Variability in definitions and criteria of extrauterine growth restriction and its association with neurodevelopmental outcomes in preterm infants: a narrative review. Nutrients. 2024;16:968. https://doi.org/10.3390/nu16070968
- Martínez-Jiménez MD, Gómez-García FJ, Gil-Campos M, Pérez-Navero JL. Comorbidities in childhood associated with extrauterine growth restriction in preterm infants: a scoping review. Eur J Pediatr. 2020;179:1255-65. https://doi.org/10.1007/s00431-020-03613-8
- Franz AR, Pohlandt F, Bode H, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics. 2009;123:e101-9. https://doi.org/10.1542/peds.2008-1352
- Griffin IJ, Tancredi DJ, Bertino E, Lee HC, Profit J. Postnatal growth failure in very low birthweight infants born between 2005 and 2012. Arch Dis Child Fetal Neonatal Ed. 2016;101:F50-5. https://doi.org/10.1136/archdischild-2014-308095
- Figueras-Aloy J, Palet-Trujols C, Matas-Barceló I, Botet-Mussons F, Carbonell-Estrany X. Extrauterine growth restriction in very preterm infant: etiology, diagnosis, and 2-year follow-up. Eur J Pediatr. 2020;179:1469-79. https://doi.org/10.1007/s00431-020-03628-1
- World Health Organization. Preterm birth factsheet. 2018. Available at: https://www.who.int/news-room/fact-sheets/detail/preterm-birth
- Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. https://doi.org/10.1186/1471-2431-13-59
- Patel AL, Engstrom JL, Meier PP, Kimura RE. Accuracy of methods for calculating postnatal growth velocity for extremely low birth weight infants. Pediatrics. 2005;116:1466-73. https://doi.org/10.1542/peds.2004-1699
- Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1-7. https://doi.org/10.1097/00000658-197801000-00001
- Koç E, Baş AY, Özdek Ş, Ovalı F, Başmak H. Turkish Neonatal and Turkish Ophthalmology Societies consensus guideline on the retinopathy of prematurity. Turk Pediatri Ars. 2018;53:S151-60. https://doi.org/10.5152/TurkPediatriArs.2018.01815
- Higgins RD, Jobe AH, Koso-Thomas M, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. 2018;197:300-8. https://doi.org/10.1016/j.jpeds.2018.01.043
- Satar M, Arısoy AE, Çelik İH. Turkish Neonatal Society guideline on neonatal infections-diagnosis and treatment. Turk Pediatri Ars. 2018;53:S88-100. https://doi.org/10.5152/TurkPediatriArs.2018.01809
- Lin Z, Green RS, Chen S, et al. Quantification of EUGR as a measure of the quality of nutritional care of premature infants. PLoS One. 2015;10:e0132584. https://doi.org/10.1371/journal.pone.0132584
- Koletzko B, Poindexter B. Nutrition care of preterm infants, scientific basis and practical guidelines. Skarger Pub. 2014:277. https://doi.org/10.1159/isbn.978-3-318-02641-2
- Simon L, Hanf M, Frondas-Chauty A, et al. Neonatal growth velocity of preterm infants: the weight Z-score change versus Patel exponential model. PLoS One. 2019;14:e0218746. https://doi.org/10.1371/journal.pone.0218746
- De Rose DU, Cota F, Gallini F, et al. Extra-uterine growth restriction in preterm infants: neurodevelopmental outcomes according to different definitions. Eur J Paediatr Neurol. 2021;33:135-45. https://doi.org/10.1016/j.ejpn.2021.06.004
- Shen W, Wu F, Mao J, et al. Analysis of "true extrauterine growth retardation" and related factors in very preterm infants-A multicenter prospective study in China. Front Pediatr. 2022;10:e876310. https://doi.org/10.3389/fped.2022.876310
- Huang XR, Shen W, Wu F, et al. Real-world evidence regarding the growth of very premature infants with small for gestational age after birth: a multicenter survey in China. BMC Pediatr. 2023;23:437. https://doi.org/10.1186/s12887-023-04245-1
- Zhao T, Feng HM, Caicike B, Zhu YP. Investigation Into the current situation and analysis of the factors influencing extrauterine growth retardation in preterm infants. Front Pediatr. 2021;9:643387. https://doi.org/10.3389/fped.2021.643387
- Embleton ND, Jennifer Moltu S, Lapillonne A, et al. Enteral nutrition in preterm infants (2022): a position paper from the ESPGHAN committee on nutrition and invited experts. J Pediatr Gastroenterol Nutr. 2023;76:248-68. https://doi.org/10.1097/MPG.0000000000003642
- Brennan AM, Fenton S, Murphy BP, Kiely ME. Transition phase nutrition recommendations: a missing link in the nutrition management of preterm infants. JPEN J Parenter Enteral Nutr. 2018;42:343-51. https://doi.org/10.1177/0148607116686289
- Arslanoglu S, Boquien CY, King C, et al. Fortification of human milk for preterm infants: update and recommendations of the European Milk Bank Association (EMBA) working group on human milk fortification. Front Pediatr. 2019;7:76. https://doi.org/10.3389/fped.2019.00076
- Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics. 2003;111:986-90. https://doi.org/10.1542/peds.111.5.986
- Zozaya C, Avila-Alvarez A, Arruza L, et al. The effect of morbidity and sex on postnatal growth of very preterm infants: a multicenter cohort study. Neonatology. 2019;115:348-54. https://doi.org/10.1159/000497221
- Greenbury SF, Angelini ED, Ougham K, et al. Birthweight and patterns of postnatal weight gain in very and extremely preterm babies in England and Wales, 2008-19: a cohort study. Lancet Child Adolesc Health. 2021;5:719-28. https://doi.org/10.1016/S2352-4642(21)00232-7
- Moltu SJ, Bronsky J, Embleton N, et al. Nutritional management of the critically ill neonate: a position paper of the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2021;73:274-89. https://doi.org/10.1097/MPG.0000000000003076
Copyright and license
Copyright © 2025 The author(s). This is an open-access article published by Aydın Pediatric Society under the terms of the Creative Commons Attribution License (CC BY) which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.