Abstract
Objective: Sodium Lauryl Sulfate (SLS), a synthetic detergent commonly used in toothpaste, has been implicated in various studies as a cause or trigger of Recurrent Oral Ulcers (ROU). This study aimed to assess the efficacy of SLS-free toothpaste recommendations in pediatric cases with isolated ROU and Behçet Disease (BD). As a secondary aim, we also evaluated whether BD developed during the follow-up of cases with ROU.
Method: This is a retrospective cohort study. Patients who received SLS-free oral care recommendations due to ROU or BD and had at least six months of follow-up data were included between February 2023 and August 2023. The clinicians did not recommend specific brands for the SLS-free oral care products. The follow-up parameters included the visual analog scale (VAS, 0-10), the total number of attacks over three months, the average number of ulcers per attack, and the average duration.
Results: The follow-up data included 78 patients with ROU and 14 patients diagnosed with BD. The mean follow-up duration was 23 ± 7 months. None of the patients referred for ROU developed BD during the follow-up. The initial VAS scores, number of attacks, number of ulcers per attack, and attack duration were higher in BD patients than in ROU patients. During the follow-up visit after initiating SLS-free toothpaste (at least three months), 38 (48.7%) ROU patients reported lower VAS scores. Six patients with BD reported a decrease in the frequency of attacks. However, there were no statistically significant differences between the baseline and 6-month data regarding the number of ulcers, VAS scores, number of attacks, or attack duration.
Conclusion: SLS-free toothpaste could be a good recommendation for ROU cases. Although our limited number of BD cases did not yield statistically significant results, as previously mentioned, some patients reported lower pain scores and fewer attacks.
Keywords: sodium lauryl sulfate, aphthous ulcer
INTRODUCTION
Recurrent oral ulcer (ROU) is the most common inflammatory disease of the oral mucosa, affecting 5%-25% of the pediatric population.1 It is characterized by one or more painful ulcers covered with a white or grayish pseudomembrane and surrounded by a well-defined erythematous halo. These ulcers typically heal spontaneously within 4-7 days.2 However, their painful nature can impair speaking, swallowing, and chewing, thereby affecting quality of life. The etiology of ROU has not yet been clarified. Immune issues, vitamin deficiencies, stress, local trauma, infections, and diet are implicated in its etiology.3 The high prevalence of ROU in the parents of children with ROU suggests a potential genetic predisposition. ROU is also a common reason for referral to pediatric rheumatology clinics because it can be a symptom of rheumatologic diseases like Behçet’s disease (BD) and Periodic Fever, Aphthous Stomatitis, Pharyngitis, and Cervical Adenitis (PFAPA) syndrome. Increasing shared single nucleotide polymorphisms (SNPs) in ROU, PFAPA, and BD indicate that some cases may be on a shared spectrum.4 Although variable rates have been reported in the literature, recurrent oral aphthae in BD have been reported with a rate of 97%.5 However, ROU is rarely caused by underlying BD, and PFAPA is distinguished from isolated ROU by its characteristic features.
Sodium Lauryl Sulfate (SLS), a synthetic detergent commonly used in toothpaste, has been implicated in various studies as a cause or trigger of ROU.6 It is thought to increase the penetration of exogenous antigens into the oral mucosa by affecting the barrier function.7 It is also known to cause contact dermatitis by increasing epidermal water loss. The literature contains conflicting results from studies evaluating the frequency of ROU with SLS-free toothpaste. However, the efficacy of SLS-free toothpaste recommendations has yet to be evaluated in BD. In this study, we aimed to assess the efficacy of SLS-free toothpaste recommendations in pediatric cases with isolated ROU and BD. As a secondary aim, we also evaluated whether BD developed during the follow-up of cases with ROU.
MATERIAL AND METHODS
This study is a retrospective cohort analysis. Patients who received SLS-free oral care recommendations due to ROU or BD and had at least six months of follow-up data were included between February 2023 and August 2023. BD patients met the PEDBD (Pediatric Behçet’s Disease Criteria)8 and ICBD9 (International Criteria for Behçet’s Disease). Before diagnosing recurrent oral ulcers (ROU), the following conditions were excluded based on clinical examination, patient history, or laboratory tests: (Immunological deficiencies, hormonal disorders, hematological deficiencies, trauma, medications, microorganisms, oral streptococci, helicobacter pylori (H. pylori), herpes viruses). The clinicians did not recommend specific brands for the SLS-free oral care products. Patients with ROU who simultaneously started vitamin replacement therapy due to deficiencies were excluded. Additionally, cases in which patients had never brushed their teeth or brushed irregularly were excluded. Only BD patients received colchicine treatment. No other medication was used in both groups. Those who had a simultaneous colchicine dose adjustment or were initiated on immunosuppressive therapy to their treatment were excluded. Incomplete patients were excluded from the study. The follow-up parameters included the visual analog scale (VAS, 0-10), the total number of attacks over three months, the average number of ulcers per attack, and the average duration. The follow-up frequency for patients with BD was every three months. Patients with ROU had follow-up visits every three months. The aim was to evaluate the baseline and 6th-month data for patients with BD and the baseline, 3rd-month, and 6th-month data for patients with ROU.
Statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 26.0, and JASP Version 0.18.3. The normality of the data was assessed using graphical methods, the Shapiro-Wilk test, and the Kolmogorov-Smirnov test, while Levene’s test was used to determine the homogeneity of variances. Non-parametric tests were utilized for non-normally distributed data. Descriptive statistics included median and interquartile range (IQR) for continuous variables and frequency and percentage for categorical variables. The Mann-Whitney U test compared two independent groups, the Wilcoxon signed-rank test compared baseline and follow-up measurements in the same sample, and the Friedman test evaluated more than two related groups. Post-hoc analyses, employing the Wilcoxon signed-rank test with Bonferroni correction, were conducted when the Friedman test indicated significant differences. A significance level of p<0.05 was used for all statistical analyses. The Ethics Committee approved the study, and written informed consent was obtained from the patients or parents per the Helsinki Declaration.
RESULTS
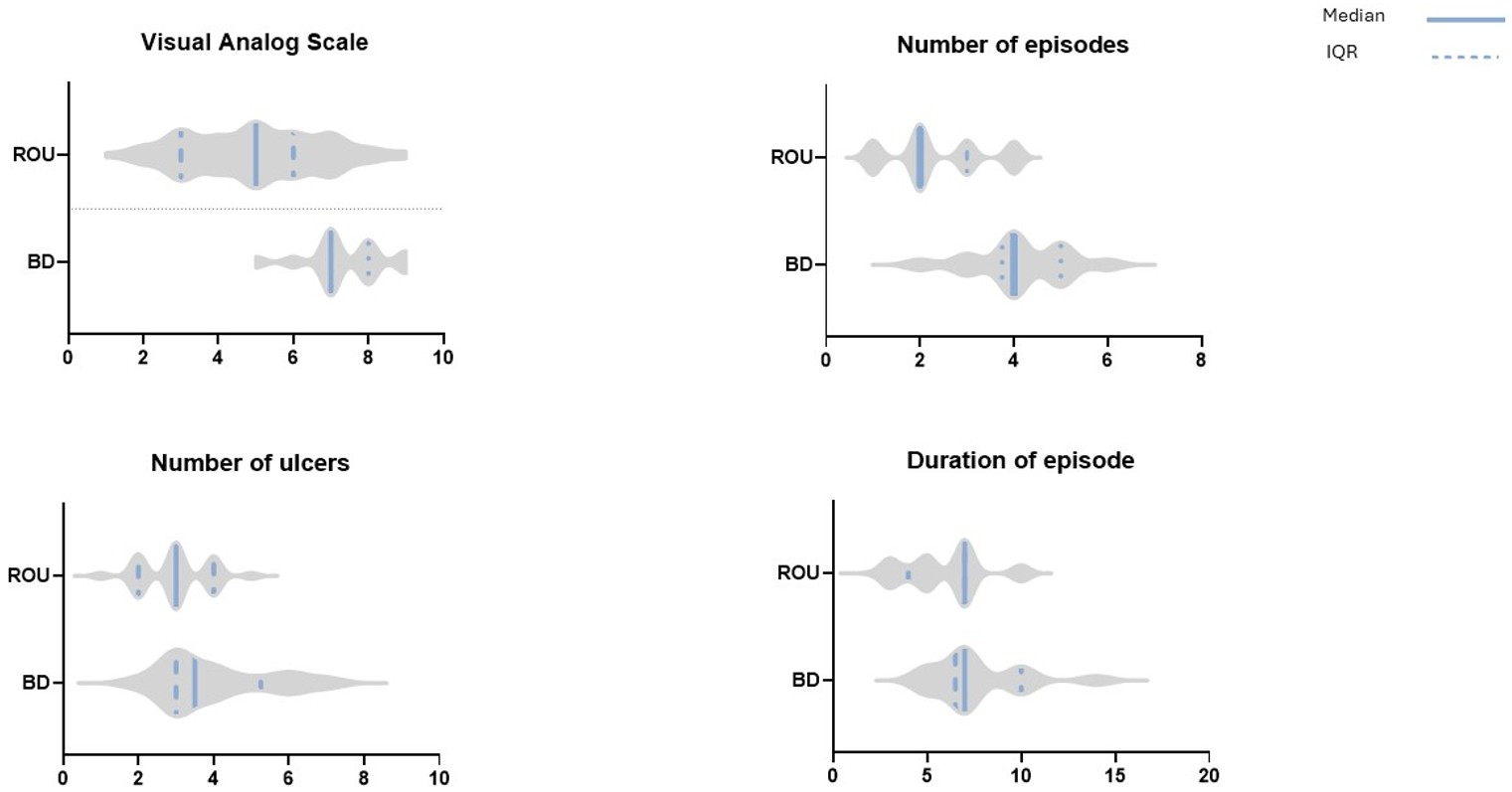
The follow-up data included 78 patients with ROU and 14 patients with BD. Among the ROU patients, 35 (44.9%) were female. The mean age at presentation was 9.1 ± 3.4 years. Among the patients diagnosed with BD, nine were male, and five were female. The mean age was 13 ± 3.7 years. All patients had oral aphthae, genital aphthae, and skin findings (erythema nodosum), with no additional findings. The minimum follow-up duration was nine months, and the maximum was 37 months. None of the patients referred for ROU developed BD during the follow-up. The initial VAS scores, number of attacks, number of ulcers per attack, and attack duration were higher in BD patients than in ROU patients (Table 1, Figure 1).
| ROU: recurrent oral ulcers; BD: Behçet disease | |||
| Table 1. The patients' initial VAS scores, frequency of attacks, number of ulcers per attack, and duration of each attack | |||
| Variable | Group | n | Mean Rank / P-value |
| Visual Analog Scale | ROU | 78 | 41.46 |
| BD | 14 | 74.57 | |
| p < 0.001* | |||
| Number of Episodes | ROU | 78 | 41.26 |
| BD | 14 | 76.68 | |
| p < 0.001* | |||
| Number of Ulcers | ROU | 78 | 43.75 |
| BD | 14 | 61.82 | |
| p = 0.014* | |||
| Duration of Episode | ROU | 78 | 43.71 |
| BD | 14 | 62.04 | |
| p = 0.013* | |||
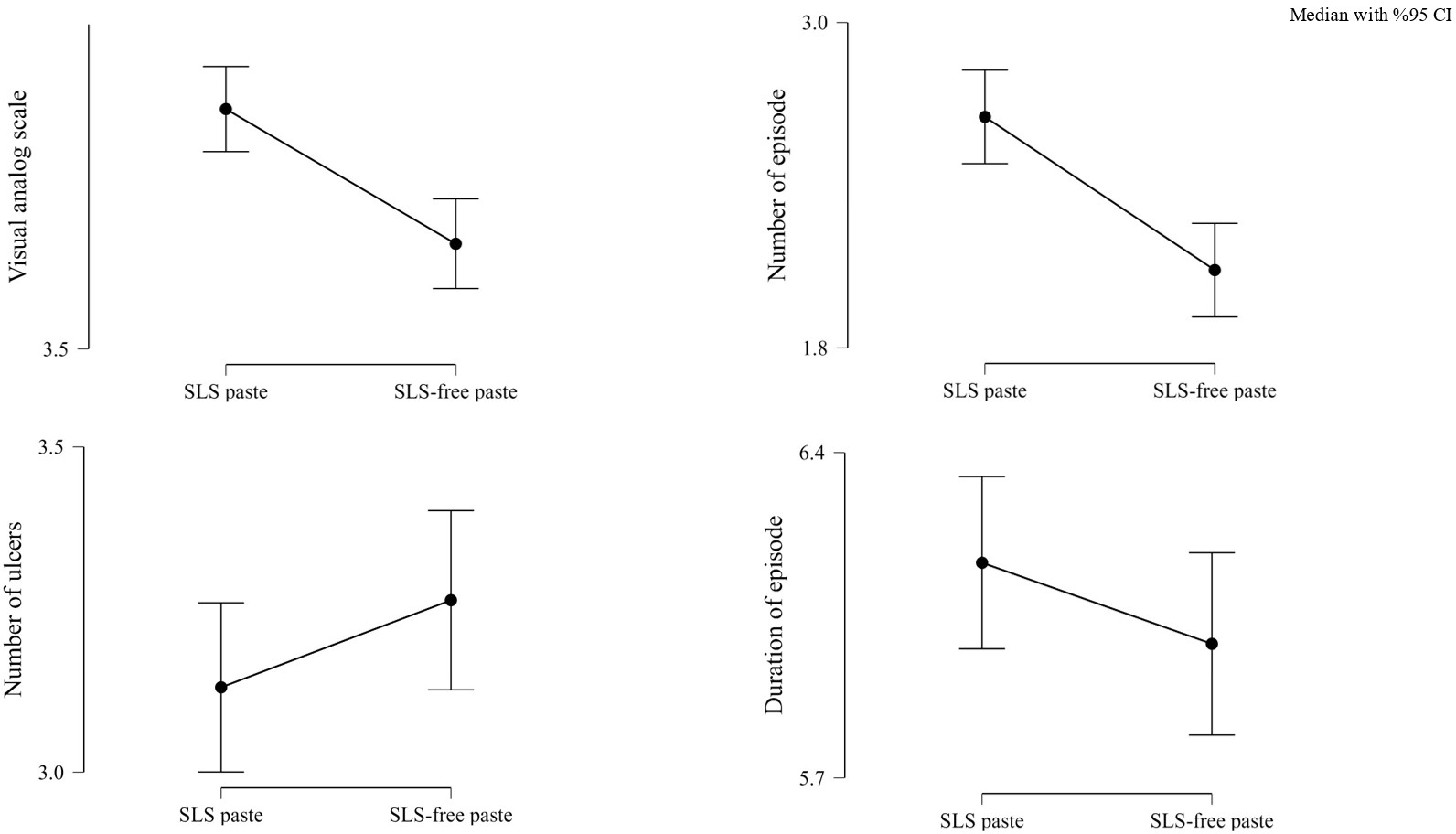
During the follow-up visit after initiating SLS-free toothpaste (at least three months), 38 (48.7%) ROU patients reported reduced VAS scores. Additionally, 42 (53.8%) patients had fewer attacks over the three months. The decrease in VAS scores and attacks in the third month was statistically significant. In contrast, the average number of ulcers per attack and the attack duration remained unchanged (Table 2, Figure 2).
|
SLS: sodium lauryl sulfate *P value was considered significant when < 0.05. |
|||||
| Table 2. Baseline and 3-month parameters of patients with recurrent oral aphthae | |||||
| Variable | Group | n | IQR | Mean ± SD | p-value |
| Visual Analog Scale | SLS Paste | 78 | 5 (3-6) | 4.99 ± 1.82 | <0.01* |
| SLS-free Paste | 78 | 2 (3-5) | 3.78 ± 1.95 | ||
| Number of Episodes | SLS Paste | 78 | 2 (2-3) | 2.38 ± 1.02 | <0.01* |
| SLS-free Paste | 78 | 2 (1-3) | 1.87 ± 0.97 | ||
| Number of Ulcers | SLS Paste | 78 | 3 (2-4) | 2.97 ± 0.91 | 0.483 |
| SLS-free Paste | 78 | 3 (3-4) | 3.14 ± 0.87 | ||
| Duration of Episode | SLS Paste | 78 | 7 (4-7) | 5.88 ± 2.09 | 0.187 |
| SLS-free Paste | 78 | 5 (4-7) | 5.68 ± 2.17 | ||
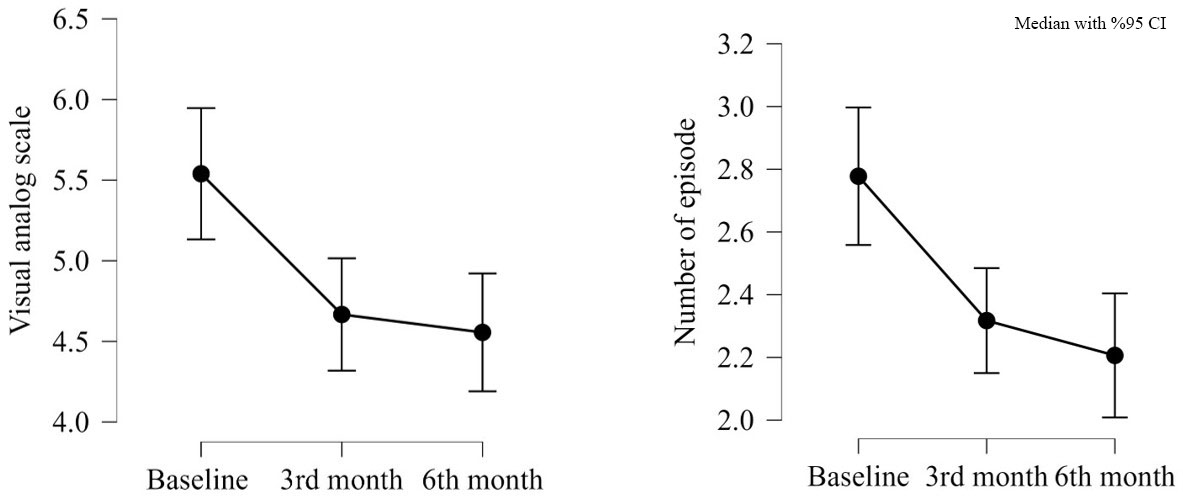
Twenty-nine patients were lost to follow-up the 6-month follow-up after the initial change. Of these, 15 patients reported reductions in VAS scores and the number of attacks at the 3-month follow-up. Five patients had a reduction in VAS scores, while nine patients showed no decrease in either attack frequency or VAS scores. Of the 49 patients with follow-up data, 13 experienced a reduction in VAS scores during the follow-up despite no reduction in the first three months. Six patients reported lower VAS scores at the 6-month follow-up than the 3-month follow-up. When comparing VAS scores at baseline, three months, and six months, the 6-month scores were significantly lower than the baseline scores. The number of attacks also significantly decreased between the baseline and 6-month follow-up data (Table 3, Figure 3). The number of ulcers per attack and attack duration were not reassessed for these patients.
|
M: months; a: baseline; b: 3 months; c: 6 months Pairwise comparisons: Baseline, 3 Months, and 6 Months. Significant differences are marked with * (Bonferroni-adjusted p < 0.05). Non-significant comparisons are also shown for completeness. *P value was considered significant when < 0.05. |
||||||
| Table 3. Baseline, 3-month, and 6-month parameters of patients with recurrent oral aphthae | ||||||
| Variable | n | Friedman p-value | Pairwise Comparisons (Wilcoxon) | Wilcoxon Test (Wi) | p-value | Bonferroni Adjusted p-value |
| Visual Analog Scale | 49 | 0.001* | Baseline | 99.500, | 0.006a-b* | 0.017 a-b* |
| 3M | 149.000 | 0.001a-c* | 0.004 a-c* | |||
| 6M | 252.000 | 0.606b-c | 1.000 b-c | |||
| Number of Episodes | 49 | 0.016* | Baseline | 198.000 | 0.023a-b* | 0.070a-b* |
| 3M | 89.000, | 0.004a-c* | 0.012a-c* | |||
| 6M | 95.500 | 0.160b-c | 0.480b-c | |||
Six patients with BD reported a decrease in the frequency of attacks. However, there were no statistically significant differences between the baseline and 6-month data regarding the number of ulcers, VAS scores, number of attacks, or attack duration (Table 4).
|
SLS: sodium lauryl sulfate *P value was considered significant when < 0.05. |
|||||
| Table 4. Baseline and 6-month data on the number of ulcers, VAS scores, frequency of attacks, or attack duration in Behçet's patients | |||||
| Variable | Group | n | IQR | Mean ± SD | p-value |
| Visual Analog Scale | SLS Paste | 78 | 5 (3-6) | 4.99 ± 1.82 | <0.01* |
| SLS-free Paste | 78 | 2 (3-5) | 3.78 ± 1.95 | ||
| Number of Episodes | SLS Paste | 78 | 2 (2-3) | 2.38 ± 1.02 | <0.01* |
| SLS-free Paste | 78 | 2 (1-3) | 1.87 ± 0.97 | ||
| Number of Ulcers | SLS Paste | 78 | 3 (2-4) | 2.97 ± 0.91 | 0.483 |
| SLS-free Paste | 78 | 3 (3-4) | 3.14 ± 0.87 | ||
| Duration of Episode | SLS Paste | 78 | 7 (4-7) | 5.88 ± 2.09 | 0.187 |
| SLS-free Paste | 78 | 5 (4-7) | 5.68 ± 2.17 | ||
DISCUSSION
Sodium Lauryl Sulfate is a detergent and foaming agent widely used in toothpaste. The foam facilitates the spreading of the toothpaste in the oral cavity and provides a sense of cleanliness.10 SLS also serves as a plaque-cleaning agent by loosening and emulsifying plaque deposits, which aids in their removal and may help prevent periodontal diseases.11 Lastly, it exhibits antimicrobial effects by disrupting bacterial cell integrity and inhibiting their metabolic activities.12
Despite these beneficial effects, the weakening of membrane integrity makes the oral mucosa more vulnerable to trauma and irritants. This can lead to apoptosis and inflammatory reactions.13 In patients with ROU, elevated levels of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 have been observed, exacerbating the inflammatory response and prolonging ulcer healing.14
Mouth rinses containing concentrations higher of Sodium Lauryl Sulfate (SLS) than those found in commercially available toothpaste have been shown to cause prolonged burning sensations and mucosal erosion. Barkvoll and Rolla were the first to demonstrate in the literature that using SLS-free toothpaste significantly reduced ulcer incidence.15 In a randomized controlled trial, the use of SLS-free toothpaste was associated with lower pain scores and ulcer incidence; however, no changes were observed in ulcer count or healing time.16 Another study, primarily involving adult patients, found no benefits for any parameter.17 However, a systematic review analyzing the results of four studies found that SLS-free paste usage was associated with reductions in all four parameters.18
This study is the first to evaluate the effectiveness of SLS-free toothpaste in pediatric BD and ROU. Due to the high prevalence of BD in Turkey, oral aphthae in pediatric cases are a significant source of stress for families. During our 9–37-month follow-up period, patients referred for isolated ROU did not develop other clinical features of BD. Notably, patients with BD (though the number of observations was limited) reported more frequent attacks, a higher number of ulcers, and higher VAS scores.
While nearly half of the patients reported lower attack frequency and VAS scores, the number of ulcers and the duration of attacks did not change, suggesting that SLS might contribute as a potential exacerbating factor in the multifactorial process.
CONCLUSIONS
SLS-free toothpaste may be recommended for ROU cases. Although our limited number of BD cases did not yield statistically significant results, as previously mentioned, some patients reported lower pain scores and fewer attacks. It is not a medication and is cost-effective, so it can be tried while adhering to existing treatment algorithms. Well-designed studies are warranted for BD cases as well. Clinicians recommended SLS-free oral care without endorsing a specific brand, and patients might have eliminated other toothpaste ingredients through different brand preferences. This was not scrutinized in our routine evaluations. The small number of patients, particularly in the BD cohort, might have prevented the detection of potential clinical efficacy statistically. Due to the retrospective nature of the study, there is a possibility of recall bias.
Ethical approval
This study has been approved by the Hacettepe University Health Sciences Research Ethics Committee (approval date 08.10.2024, number 2024/17-48). Written informed consent was obtained from the participants.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Sánchez-Bernal J, Conejero C, Conejero R. Recurrent aphthous atomatitis. Actas Dermosifiliogr. 2020;111:471-80. https://doi.org/10.1016/j.ad.2019.09.004
- Cui RZ, Bruce AJ, Rogers RS. Recurrent aphthous stomatitis. Clin Dermatol. 2016;34:475-81. https://doi.org/10.1016/j.clindermatol.2016.02.020
- Akintoye SO, Greenberg MS. Recurrent aphthous stomatitis. Dent Clin North Am. 2014;58:281-97. https://doi.org/10.1016/j.cden.2013.12.002
- Manthiram K, Preite S, Dedeoglu F, et al. Common genetic susceptibility loci link PFAPA syndrome, Behçet's disease, and recurrent aphthous stomatitis. Proc Natl Acad Sci U S A. 2020;117:14405-11. https://doi.org/10.1073/pnas.2002051117
- Bozkurt T, Yıldız M, Deniz R, et al. Clinical course of pediatric-onset Behçet's disease in young adulthood. Rheumatology (Oxford). 2024. https://doi.org/10.1093/rheumatology/keae624
- Fakhry-Smith S, Din C, Nathoo SA, Gaffar A. Clearance of sodium lauryl sulphate from the oral cavity. J Clin Periodontol. 1997;24:313-7. https://doi.org/10.1111/j.1600-051x.1997.tb00763.x
- Sabri H, Derakhshan Barjoei MM, Azarm A, et al. The yin and yang of sodium lauryl sulfate use for oral and periodontal health: a literature review. J Dent (Shiraz). 2023;24:262-76.
- Koné-Paut I, Shahram F, Darce-Bello M, et al. Consensus classification criteria for paediatric Behçet's disease from a prospective observational cohort: PEDBD. Ann Rheum Dis. 2016;75:958-64. https://doi.org/10.1136/annrheumdis-2015-208491
- International Team for the Revision of the International Criteria for Behçet's Disease (ITR-ICBD). The International Criteria for Behçet's Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28:338-47. https://doi.org/10.1111/jdv.12107
- Healy CM, Paterson M, Joyston-Bechal S, Williams DM, Thornhill MH. The effect of a sodium lauryl sulfate-free dentifrice on patients with recurrent oral ulceration. Oral Dis. 1999;5:39-43. https://doi.org/10.1111/j.1601-0825.1999.tb00062.x
- Wade WG, Addy M. Antibacterial activity of some triclosan-containing toothpastes and their ingredients. J Periodontol. 1992;63:280-2. https://doi.org/10.1902/jop.1992.63.4.280
- Giertsen E, Scheie AA, Rölla G. Plaque inhibition by a combination of zinc citrate and sodium lauryl sulfate. Caries Res. 1989;23:278-83. https://doi.org/10.1159/000261192
- Natah SS, Konttinen YT, Enattah NS, Ashammakhi N, Sharkey KA, Häyrinen-Immonen R. Recurrent aphthous ulcers today: a review of the growing knowledge. Int J Oral Maxillofac Surg. 2004;33:221-34. https://doi.org/10.1006/ijom.2002.0446
- Slebioda Z, Szponar E, Kowalska A. Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: literature review. Arch Immunol Ther Exp (Warsz). 2014;62:205-15. https://doi.org/10.1007/s00005-013-0261-y
- Barkvoll P, Rolla G. Possible effects of sodium lauryl sulfate (SLS) on recurrent aphthous ulcers. J Dent Res. 1991;70:549.
- Herlofson BB, Barkvoll P. Sodium lauryl sulfate and recurrent aphthous ulcers. A preliminary study. Acta Odontol Scand. 1994;52:257-9. https://doi.org/10.3109/00016359409029036
- Shim YJ, Choi JH, Ahn HJ, Kwon JS. Effect of sodium lauryl sulfate on recurrent aphthous stomatitis: a randomized controlled clinical trial. Oral Dis. 2012;18:655-60. https://doi.org/10.1111/j.1601-0825.2012.01920.x
- Alli BY, Erinoso OA, Olawuyi AB. Effect of sodium lauryl sulfate on recurrent aphthous stomatitis: a systematic review. J Oral Pathol Med. 2019;48:358-64. https://doi.org/10.1111/jop.12845
Copyright and license
Copyright © 2025 The author(s). This is an open-access article published by Aydın Pediatric Society under the terms of the Creative Commons Attribution License (CC BY) which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.