Abstract
Objective: B-cell Non-Hodgkin Lymphoma (B-NHL) is an aggressive malignancy in children requiring prompt multidisciplinary management. This retrospective cohort study aims to evaluate the clinical characteristics, treatment outcomes, and impact of rituximab (RTX) in pediatric B-NHL patients.
Methods: We retrospectively analyzed 62 pediatric B-NHL patients treated at tertiary centers. Patient demographics, clinical presentation, histopathological subtypes, disease stage, treatment regimens, and survival outcomes were assessed. Event-free survival (EFS) and overall survival (OS) rates were analyzed based on lactate dehydrogenase (LDH) levels and RTX administration.
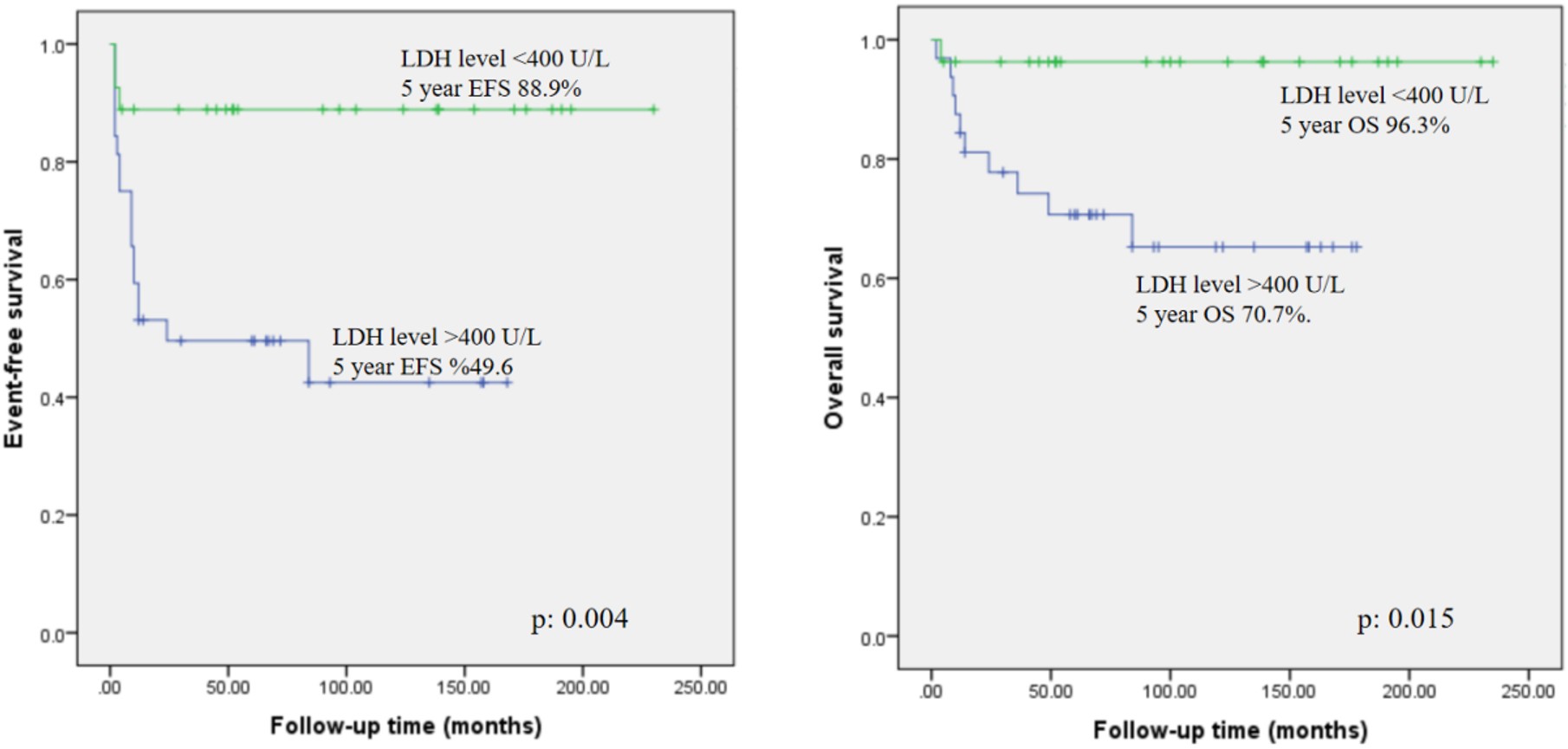
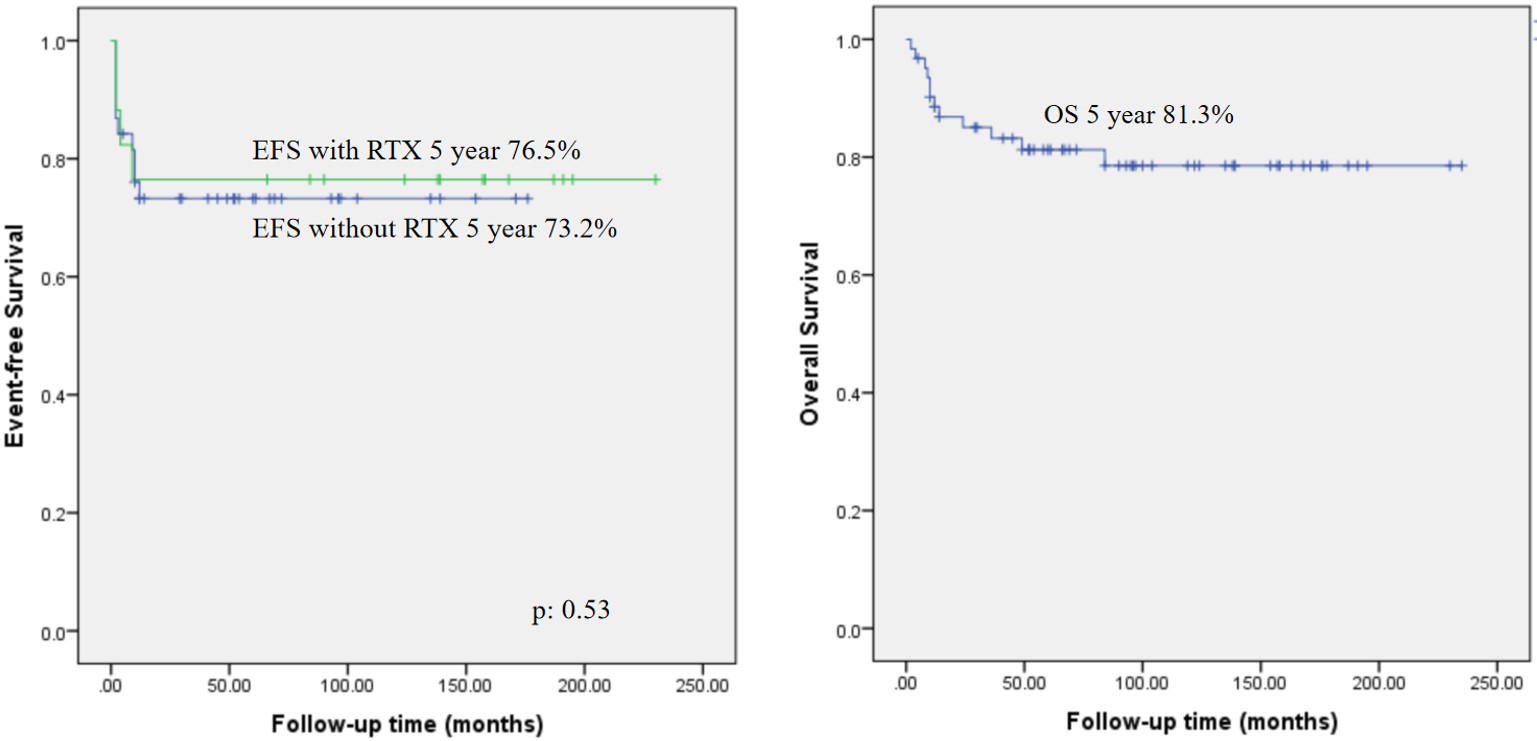
Results: The mean age at diagnosis was 8.73±4.3 years, with a male predominance (79%). The most common histological subtype was Burkitt lymphoma (BL) (53.2%), followed by diffuse large B-cell lymphoma (DLBCL) (33.8%). Advanced-stage disease (III-IV) was observed in 74.1% of cases. RTX was administered in 72.5% of patients, with a mean of 5.1±2.7 doses. Febrile neutropenia (FEN) was noted in 74.1%, with intensive care unit (ICU) admission required for seven patients. Mortality was observed in 12 (19.3%) patients, including all patients with primary immunodeficiency (PID). The 5-year EFS for the entire cohort was 67.2%, and OS was 81.3%. Patients with LDH <400 U/L had superior 5-year EFS (88.9%) and OS (96.3%) compared to those with LDH >400 U/L (EFS: 49.6%, OS: 70.7%; p=0.004 and p=0.015, respectively). In RTX-treated patients without PID, EFS was 76.5% versus 73.2% in those without RTX, but the difference was not statistically significant (p=0.53).
Conclusions: Although not statistically significant, EFS was found to be higher in the RTX-treated group, suggesting that adding RTX to standard chemotherapy regimens may improve survival, particularly for high-risk patients, though its benefit in low-risk cases remains uncertain. Despite improved survival, patients with PID had poor outcomes, likely due to increased infections and disseminated disease. Risk-adapted, targeted treatment strategies are essential for optimizing outcomes in pediatric B-NHL. Further large-scale, randomized controlled trials are needed to confirm the efficacy of RTX in different risk groups and to optimize treatment regimens for pediatric B-NHL.
Keywords: rituximab, non-hodgkin lymphoma, primary immunodeficiencies
INTRODUCTION
Non-Hodgkin lymphoma, accounting for only 7% of cancers in the pediatric population younger than 20 years old, is an important tumor due to its high grade.1 Certain variables, such as geographical location, age, gender, race, whether the patient is infected with Ebstein-Barr virus or diagnosed with an inherited disease associated with cancer development, and the patient’s immune status impact incidence.2
Despite the improved survival rates, as high as ≥95% 5-year event-free survival (EFS) rate and 80-90% 5-year overall survival (OS), the outcome depends on many factors, including stage and histology of the disease. It should be underlined that, except for anaplastic large cell lymphoma, regardless of histology, response failure to first-line therapy is an independent prognostic factor.3 In addition to this, toxicities experienced during the courses are still a matter of debate.2,4
Mature B cell lymphomas account for nodal marginal zone lymphoma (NMZL), follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), primary mediastinal (thymic) large B-cell lymphoma, ALK (anaplastic lymphoma kinase) positive large B-cell lymphoma, Burkitt Lymphoma (BL), and B-cell lymphoma unclassifiable (B-NHL, NOS). Among them, low-grade NHL, such as FL and NMZL, are rare in childhood.2 B-cell NHLs are commonly treated with regimens containing corticosteroids, high-dose methotrexate and cytarabine, alkylating agents, prophylactic/intense (in the presence of central nervous system involvement) intrathecal injections. The protocols frequently consist of short, however intensive chemotherapy cycles. Therefore, the frequency of short-term toxicities is increased for patients with B-NHL. Although long-term toxicity incidence is still acceptable, precautions should be undertaken to reduce the risk of cardiovascular complications and secondary malignancies.5,6
In recent years, Rituximab (RTX) (Anti-CD20 monoclonal antibody) has been proven to have an efficacy both on EFS and OS in large-scale phase 3 trials, whilst it has been utilized in the standard care of patients with B-NHL in adulthood, combined with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) regimen.7 The present study is designed as a retrospective cohort evaluating the effects of RTX on both EFS and OS.
MATERIAL AND METHOD
In the current study, 62 patients diagnosed with B-NHL who had been admitted to the Pediatric Hematology and Oncology Departments of Erciyes, Van Yüzüncü YIl, Sakarya, Dicle, Hasan Kalyoncu Universities and Gaziantep City Hospital between the years 2005 and 2024 were enrolled in the study. The demographical data, stage, and histology of the disease, treatment regimens, and treatment outcomes were obtained from the patient files. Besides, the frequency of treatment-related complications, for example, febrile neutropenia and fungal infections, intensive care unit admission, and the necessity of granulocyte-macrophage colony-stimulating factor and/or immunoglobulin utilization in the periods of severe infections were evaluated.
The staging and risk group stratification were assessed in accordance with the NHL-BFM protocol designed by the Berlin-Frankfurt-Münster Group. According to this protocol, risk group stratification depends on the resection status of the tumor, stage, and serum lactate dehydrogenase (LDH) level on admission. The staging system is primarily based on St Jude’s staging.8-10 R1 risk group is defined as the completely resected tumor, R2 is defined as stage I or II disease with an incomplete resected tumor, or stage III disease with an LDH level of <500 U/L. Whereas R3 is defined as stage III disease with an LDH level equal to or higher than 500 U/L and lower than 1000 U/L, or stage IV Burkitt Leukemia with an LDH level <1.000 U/L and no involvement of central nervous system (CNS). R4 is defined as stage III disease with an LDH level of >1.000 U/L or stage IV Burkitt leukemia with an LDH level of ≥1.000 U/L in the presence of CNS negativity. In the presence of CNS involvement, cases are classified as R4 CNS+.8-10
The primary endpoint of this retrospective cohort was to achieve information on event-free (EFS) and overall survival rates (OS). Besides, the effect of monoclonal anti-CD20 antibody RTX on survival and side effects was evaluated.
Continuous data were compared using the Student t-test if normally distributed; if not, they were compared using the Mann-Whitney U test. Also, proportions were compared with the x2 test and Fisher exact test. Survival was evaluated with Kaplan Meier and Log-rank tests. A p-value<0.05 was considered statistically significant. Results were analyzed using SPSS version 22.0 software.
RESULTS
Of the 62 patients enrolled in the study, only 13 (21%) were female and 49 (79%) were male. Regarding the subtypes of B-NHL, 33 patients (53.2%) were diagnosed with BL, 21 (33.8%) with DLBCL, 2 (3.2%) with ALBCL, and 1 (1.6%) with FL, 1 (1.6%) with plasmoblastic lymphoma, and B-cell lymphoblastic lymphoma (BCLL, NOS) with indistinguishable subgroup was detected in 4 patients (6.4%). Four patients were solid organ transplanted (3 of them liver and one kidney transplantation). Of which two were diagnosed with BL, whereas two were diagnosed with DLBCL. The mean age was 8.73 (±4.3) years, whereas the median age was 8.9 years (minimum 6 months - maximum 17 years) on admission. The most common signs and symptoms on admission were swelling in the cervical area and palpable lymphadenomegaly observed in 25 (40.3%) patients, followed by abdominal distension and pain in 27 (43.5%) patients. Of the patients admitted with cervical lymphadenomegaly, 6 had shortness of breath, 5 had cough, and 4 had hoarseness. Among the patients with abdominal symptoms, 4 of them had been admitted with the clinical findings of intussusception, requiring urgent surgical intervention.
Regarding the primary sites of disease, 31 patients (50%) had abdominal, 23 (37%) had cervical, and 8 (12.9%) had mediastinal involvement. CNS involvement was present in only 2 (3.2%) patients. 5 (8%) had bone marrow involvement. Three patients (4.8%) had both bone and bone marrow disease. In contrast, four patients (6.4%) had effusions and organ involvements like liver and kidney. Considering the stages of disease according to St Jude’s staging system; 16 patients (25.8%) had stage II, 34 (54.8%) had stage III, and 12 patients (19.3%) had stage IV disease. Seventeen patients (27.4%) were stratified in R2, 30 (48.4%) in R3, and 15 (24.2%) in R4 risk group.
Considering the treatment regimens utilized in therapy, NHL-BFM protocol was initiated in 42 patients (67.7%) based on the risk groups, EPOCH (etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin) in 5 patients (8%), CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) in 11 patients (17.7%) with primary immunodeficiency (PID), and LMB89 protocol in 4 patients (6.4%). The demographical data of the whole cohort and the exact diagnosis of the 10 (16.1%) patients with PID are available in Table 1 and Table 2, respectively.
| SD: standard deviation; NHL: non-hodgkin lymphoma; R2: risk group 2; R3: risk group 3; R4: risk group 4 | |
| Table 1. Baseline characteristics of the patients | |
| Characteristics | N: 62 (%) |
| Gender | |
| Female | 13 (21%) |
| Male | 49 (79%) |
| Age | |
| Mean±SD | 8.73 (±4.3) years |
| Median (min-max) | 8.9 years (minimum 6 months - maximum 17 years) |
| Diagnosis | |
| Burkitt Lymphoma | 33 (53.2%) |
| Diffuse large B-cell lymphoma | 21 (33.8%) |
| B-NHL, not otherwise specified | 4 (6.4%) |
| Follicular Lymphoma | 1 (1.6%) |
| Plasmoblastic Lymphoma | 1 (1.6%) |
| Risk group | |
| R2 | 17 (27.4%) |
| R3 | 30 (48.4%) |
| R4 | 15 (24.2%) |
| Stage of disease (Murphy) | |
| Stage II | 16 (25.8%) |
| Stage III | 34 (54.8%) |
| Stage IV | 12 (48.4%) |
| Primary site | |
| Abdomen | 31 (50%) |
| Mediastinum | 8 (12.9%) |
| Cervical lymph nodes | 23 (37%) |
| BL: burkitt lymphoma; DLBCL: diffuse large B cell lymphoma | ||
| Table 2. Diagnosis of patients with primary immunodeficiencies | ||
| No | Primary Diagnosis | Type of NHL |
| 1 | Nijmegen Breakage Syndrome | BL |
| 2 | XLF deficiency | DLBCL |
| 3 | CD137 deficiency | DLBCL |
| 4 | TNFRSF13B mutation | DLBCL |
| 5 | MSH6 mutation | DLBCL |
| 6 | FCHO1 deficiency | DLBCL |
| 7 | FCHO1 deficiency | DLBCL |
| 8 | RASGRP1 mutation | DLBCL |
| 9 | DIAPH1-Deficiency | Plasmoblastic Lymphoma |
| 10 | IL-2 gamma receptor deficiency | DLBCL |
RTX was utilized in 45 (72.5%) patients with a mean number of applications of 5.1±2.7 times and a median of 5 times (minimum 1- maximum 17 times) with the dose of 375 mg/m.2 All the patients with PID received RTX. Before the intravenous administration of RTX, premedication with antihistamine and analgesic drugs was utilized. No adverse events related to administration were observed. Twenty-three patients (51.1%) had intravenous immunoglobulin (IVIG) in addition to RTX treatment.
Regarding the laboratory evaluation on admission, five patients (8%) had leukopenia with WBC <5000/ mm3 and three patients (4.8%) had PLT levels < 100,000/ mm3. Neutropenia was present in 2 patients (3.2%) and lymphopenia in 6 patients (9.6%). Impaired renal functions were detected in only two patients (3.2%) and elevation in transaminases in 12 (19.3%) patients. Mean LDH level was 655 U/L ±915 U/L and median 428 U/L (minimum 191 U/L- maximum 6564 U/L). The mean uric acid level was 5.6 mg/dl ±3.7 mg/dl, and the median was 5.4 mg/dl (minimum 1mg/dl- maximum 29 mg/dl). Regarding LDH levels, patients with LDH levels below 400 U/L (n:27, 43.5%) had 5-year EFS and OS of 88.9% and 96.3%, respectively. Whereas patients with LDH levels >400 U/L (n:32, 51.6%) had 5-year EFS of 49.6% and OS of 70.7%. The differences between the groups were statistically significant, with p level of 0.004 for EFS and 0.015 for OS, and available in Figure 1.
Febrile neutropenia (FEN) was determined in 46 patients (74.1%). The mean number of FEN episodes was 2.8±1.2, and the median number was 1 (minimum 0- maximum 4). Seven patients with FEN had intensive care unit (ICU) admission. Documented fungal infections were observed in 3 of the patients. One of them was the patient with PID who died owing to Candida parapsilosis sepsis. The second patient’s primary diagnosis was cystic fibrosis (CF), and had recovered from DLBCL. Candida albicans sepsis resulted in the mortality of this patient. The third one was diagnosed with BL; no underlying disease was present. The blood cultures resulted in Candida parapsilosis in this patient as well.
Twelve of the patients (19.3%) died. All of these patients had RTX in treatment. Of them, seven patients had the diagnosis of primary immunodeficiency, and one was presented as a posttransplant lymphoproliferative disease owing to immunosuppression after liver transplant. Regarding the rest of the patients, 2 of them had disseminated disease with bone and bone marrow involvement, 1 CNS involvement, and 1 had refractory ALBCL.
5-year EFS for the whole group was 67.2%, whereas 76.5% in the group received RTX, excluding the patients with PID and 73.2% who did not. The difference was not statistically significant, with a p-value of 0.53. The mean follow-up time was 7.2 (±5.3) years. 5-year OS was 81.3% in the whole cohort. Survival analyses are available in Figure 2.
DISCUSSION
Rituximab has been widely recognized for its efficacy in treating B-NHL, significantly improving OS and EFS rates in various histological subtypes. B-NHL, one of the highest-grade diseases in childhood, needs prompt management with a multidisciplinary approach. The mean age at onset is reported to be around 10 years in literature, which was 8.73 years for our retrospective cohort.10 Our cohort demonstrated a male dominancy, similar to the literature, except for primary mediastinal B-cell Lymphoma.2,10 The effects of age and gender have been evaluated in large-scale cohorts in literature. These studies disclosed that age and gender have impacts on both OS and EFS based on the histological subtype.11
In literature, both acquired and inherited immunodeficiencies are disclosed to be related to malignancy predisposition. Regarding the PIDs, NHL is the most common malignancy, followed by Hodgkin Lymphoma.12-18 Comparing the immunocompetent patients, patients with underlying immunodeficiency have dismal survival rates. However, in the era of targeted therapies and monoclonal antibodies, the survival rates and toxicities related to chemotherapy courses have been improved.19,20 Our results in the present cohort are compatible. The patients enrolled in the study with PID had a quite high mortality rate even with the treatment of RTX. These results can be attributed to the high rates of febrile neutropenia episodes, delayed diagnosis, and disseminated disease.13-18
In the present study, the most frequent pathologic subtype was BL, which is compatible with the literature.10 Besides, as expected, the most common site of BL was the abdomen, detected in 23/33 cases (69.6%). Most of our patients were diagnosed at advanced stages, such as stage III and IV determined in 46 patients (74.1%). This can be attributed to our small sample size, which cannot represent the national data. Besides, since the contributing centers are tertiary reference centers, patients with advanced diseases were consulted.
Over the last century, survival rates of mature B-cell NHL have improved significantly, with an OS of over 90%.21 The prognostic factors that have a dismal effect on survival account for high-stage, elevated LDH levels and involvement of bone marrow and/or CNS on admission. Besides, unresponsiveness to therapy is a poor prognostic factor. RTX has been proven efficient in treating adults NHL and is considered a standard of care.21,22 However, the prognosis and response rates can differ in adults and children, depending on molecular anomalies or different subtypes arising in different ages. Also, compared to adults, treatment with chemotherapy alone still has superior outcomes in children and adolescents. Despite a better prognosis, life-threatening complications may arise due to intensive chemotherapy regimens, resulting in treatment failure. Besides, RTX is a highly specific and sensitive monoclonal antibody against CD20 antigen, which is expressed in the mature B cells homogeneously. Owing to these reasons, RTX has been evaluated in the first-line therapy in addition to chemotherapy with reduced intensity both in terms of efficacy and toxicities in childhood.23,24 In our retrospective cohort study, RTX was administered as an adjunct to standard treatment in high-risk patients to facilitate rapid and effective disease control and in conjunction with low-intensity chemotherapy in immunocompromised patients to achieve disease control. Minard-Colin et al.21,22 conducted a large-scale, international, randomized phase 3 trial and disclosed that integration of RTX to standard lymphoma regimens provides a survival advantage in both EFS and OS, especially in high-risk patients defined as stage III disease with an elevated LDH or stage IV disease.22-24 Consistently, the efficacy and safety of rituximab in combination with LMB chemotherapy for children with high-risk mature B-NHL have been further supported by a Japanese multicenter trial, demonstrating a three-year event-free survival of 97.7% and an overall survival of 100%, reinforcing the robustness of RTX-based regimens in diverse populations.25 Although not statistically significant, an EFS advantage can be attributed to the RTX in the current retrospective cohort when the patients with PID are excluded. On the other hand, adverse events, hypogammaglobulinemia, and infectious episodes were increased in the RTX arm, which is not comparable with our cohort.21-24 Prior to this phase 3 international trial, a phase 2 study was conducted to search for the effect of utilizing RTX alone in newly diagnosed patients. It was disclosed that 21% of the patients responded within the 5 days before the chemotherapy regimen was initiated.23 Also, primary mediastinal B-cell lymphomas have a dismal outcome compared to other B-cell NHL subtypes. Recently, Knörr et al. disclosed that implementing RTX with the EPOCH regimen has resulted in better survival rates. On the other hand, owing to the CNS relapses in their cohort, they addressed that large-scale randomized, controlled, and collaborative trials are needed to prevent CNS disease and improve outcomes.8 Despite the promising and significantly improved outcomes, the accessibility can be a limitation owing to the economic burden on individual centers, highlighting a critical challenge in the broader implementation of this treatment in low- and middle-income countries.26
Standard and low-risk NHL patients have almost 97% OS after chemotherapy alone, which is a great response, so initiation of RTX in these patients is debated owing to reported increased adverse events.23,24 However, in our small-scale retrospective cohort, no elevation in adverse events was observed. Despite the fact that our study has major limitations, such as its retrospective nature and insufficient number of patients, it is obvious that RTX may has a survival advantage in the high-grade B-cell NHL. The addition of RTX to the cytotoxic treatment is safe in terms of side effects and is likely to contribute to event-free survival. Risk based and tailored treatment options are gaining importance in treating high-grade B-cell NHL.
Ethical approval
This study has been approved by the Erciyes University Faculty of Medicine (approval date 09.10.2024, number 224/07). Written informed consent was obtained from the participants.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Giulino-Roth L, Goldman S. Recent molecular and therapeutic advances in B-cell non-Hodgkin lymphoma in children. Br J Haematol. 2016;173:531-44. https://doi.org/10.1111/bjh.13969
- PDQ Pediatric Treatment Editorial Board. Childhood Non-Hodgkin Lymphoma Treatment (PDQ®): Health Professional Version. In: PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US); 2024.
- Attarbaschi A, Dworzak M, Steiner M, et al. Outcome of children with primary resistant or relapsed non-Hodgkin lymphoma and mature B-cell leukemia after intensive first-line treatment: a population-based analysis of the Austrian Cooperative Study Group. Pediatr Blood Cancer. 2005;44:70-6. https://doi.org/10.1002/pbc.20121
- Sekimizu M, Fukano R, Koga Y, et al. Rituximab-combined anthracycline-free chemotherapy in newly diagnosed paediatric and adolescent patients with non-high-risk aggressive mature B cell lymphoma: protocol for a single-arm, open-label, multicentre, phase II study (the Japan Children's Cancer Group Multicentre Trial, JPLSG B-NHL-20). BMJ Open. 2024;14:e080762. https://doi.org/10.1136/bmjopen-2023-080762
- Ehrhardt MJ, Chen Y, Sandlund JT, et al. Late health outcomes after contemporary lymphome malin de burkitt therapy for mature B-cell non-hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol. 2019;37:2556-70. https://doi.org/10.1200/JCO.19.00525
- Tacyildiz N, Çakmak HM, Ünal E, et al. Late outcomes in children and adolescents with non-Hodgkin lymphoma: a single-center experience. J Cancer Res Ther. 2022;18:712-7. https://doi.org/10.4103/jcrt.jcrt_80_21
- Cairo MS, Pinkerton R. Childhood, adolescent and young adult non-Hodgkin lymphoma: state of the science. Br J Haematol. 2016;173:507-30. https://doi.org/10.1111/bjh.14035
- Knörr F, Zimmermann M, Attarbaschi A, et al. Dose-adjusted EPOCH-rituximab or intensified B-NHL therapy for pediatric primary mediastinal large B-cell lymphoma. Haematologica. 2021;106:3232-5. https://doi.org/10.3324/haematol.2021.278971
- Metzger ML, Mauz-Körholz C. Epidemiology, outcome, targeted agents and immunotherapy in adolescent and young adult non-Hodgkin and Hodgkin lymphoma. Br J Haematol. 2019;185:1142-57. https://doi.org/10.1111/bjh.15789
- Gross TG, Kamdar KY, Bollard CM. Malignant Non-Hodgkin Lymphomas in children. In: Blaney SM, Helman LJ, Adamson PC, editors. Pizzo and Poplack's Pediatric Oncology. 8th ed. Philadelphia: Wolters Kluwer; 2021: 1331-92.
- Burkhardt B, Zimmermann M, Oschlies I, et al. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br J Haematol. 2005;131:39-49. https://doi.org/10.1111/j.1365-2141.2005.05735.x
- Özyörük D, Güzelküçük Z, Metin A, et al. Clinical profile and outcomes of primary immunodeficiency and malignancy in childhood at a tertiary oncology center in developing country. Pediatr Hematol Oncol. 2022;39:600-12. https://doi.org/10.1080/08880018.2022.2045408
- Patiroglu T, Akar HH, van der Burg M, Kontas O. A case of XLF deficiency presented with diffuse large B cell lymphoma in the brain. Clin Immunol. 2015;161:394-5. https://doi.org/10.1016/j.clim.2015.06.009
- Somekh I, Thian M, Medgyesi D, et al. CD137 deficiency causes immune dysregulation with predisposition to lymphomagenesis. Blood. 2019;134:1510-6. https://doi.org/10.1182/blood.2019000644
- Cekic S, Aydin F, Karali Y, et al. T-cell/histiocyte-rich large B-cell lymphoma in a patient with a novel frameshift MSH6 mutation. Pediatr Blood Cancer. 2023;70:e30008. https://doi.org/10.1002/pbc.30008
- Łyszkiewicz M, Ziętara N, Frey L, et al. Human FCHO1 deficiency reveals role for clathrin-mediated endocytosis in development and function of T cells. Nat Commun. 2020;11:1031. https://doi.org/10.1038/s41467-020-14809-9
- Somekh I, Marquardt B, Liu Y, et al. Novel mutations in RASGRP1 are associated with immunodeficiency, immune dysregulation, and EBV-induced lymphoma. J Clin Immunol. 2018;38:699-710. https://doi.org/10.1007/s10875-018-0533-8
- Azizoglu ZB, Babayeva R, Haskologlu ZS, et al. DIAPH1-deficiency is associated with major T, NK and ILC defects in humans. J Clin Immunol. 2024;44:175. https://doi.org/10.1007/s10875-024-01777-8
- Chu Y, Gardenswartz A, Termuhlen AM, Cairo MS. Advances in cellular and humoral immunotherapy - implications for the treatment of poor risk childhood, adolescent, and young adult B-cell non-Hodgkin lymphoma. Br J Haematol. 2019;185:1055-70. https://doi.org/10.1111/bjh.15753
- Cairo MS, Beishuizen A. Childhood, adolescent and young adult non-Hodgkin lymphoma: current perspectives. Br J Haematol. 2019;185:1021-42. https://doi.org/10.1111/bjh.15764
- Minard-Colin V, Brugières L, Reiter A, et al. Non-Hodgkin lymphoma in children and adolescents: progress through effective collaboration, current knowledge, and challenges ahead. J Clin Oncol. 2015;33:2963-74. https://doi.org/10.1200/JCO.2014.59.5827
- Minard-Colin V, Aupérin A, Pillon M, et al. Rituximab for high-risk, mature B-cell non-Hodgkin's lymphoma in children. N Engl J Med. 2020;382:2207-19. https://doi.org/10.1056/NEJMoa1915315
- Meinhardt A, Burkhardt B, Zimmermann M, et al. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin's lymphoma and Burkitt leukemia. J Clin Oncol. 2010;28:3115-21. https://doi.org/10.1200/JCO.2009.26.6791
- Samochatova EV, Maschan AA, Shelikhova LN, et al. Therapy of advanced-stage mature B-cell lymphoma and leukemia in children and adolescents with rituximab and reduced intensity induction chemotherapy (B-NHL 2004M protocol): the results of a multicenter study. J Pediatr Hematol Oncol. 2014;36:395-401. https://doi.org/10.1097/MPH.0b013e31829d4900
- Mori T, Osumi T, Kada A, et al. Rituximab with standard LMB chemotherapy in pediatric high-risk mature B-cell non-Hodgkin lymphoma: a report from the JPLSG B-NHL14 trial. Eur J Haematol. 2024;112:585-93. https://doi.org/10.1111/ejh.14148
- de Castro AAC, de Oliveira LA, de Andrade DP, Carbone EK, Rosati R. Use of rituximab in mature, high-grade and advanced-stage pediatric B-lineage non-Hodgkin lymphomas: a systematic review, meta-analysis and the Brazilian reality. Front Pediatr. 2025;13:1532274. https://doi.org/10.3389/fped.2025.1532274
Copyright and license
Copyright © 2025 The author(s). This is an open-access article published by Aydın Pediatric Society under the terms of the Creative Commons Attribution License (CC BY) which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.