Abstract
Objective: To evaluate bone mineral density (BMD) and the nutritional and biochemical factors affecting it in children with glycogen storage diseases (GSD) and organic acidemias (OA), which are rare metabolic disorders.
Methods: This retrospective study included 31 pediatric patients with genetically confirmed diagnoses—15 with GSD (types I and III) and 16 with OA (methylmalonic and propionic acidemia). BMD was assessed using dual-energy X-ray absorptiometry (DXA) and reported as age-adjusted Z-scores. Anthropometric data, three-day dietary records (analyzed with BeBIS 8.2), and serum markers, including vitamin D, Parathyroid Hormone (PTH), calcium, phosphorus, and others, were analyzed. Malnutrition and stunting were defined using World Health Organization (WHO) growth standards. Pearson correlation analysis was used, with a significance level set at p<0.05.
Results: In the GSD group, the mean DXA Z-score was −2.59±1.45, and low BMD (Z≤−2.0) was identified in 53.3% of patients. In the OA group, the mean Z-score was −1.91±1.19, with low BMD observed in 50%. Among GSD patients, DXA Z-scores correlated positively with dietary calcium intake (r=0.53, p=0.04), height-for-age Z-score (r=0.52, p=0.04), and serum vitamin D (r=0.58, p=0.02), while negative correlations were found with age (r=−0.87, p=0.00), disease duration (r=−0.87, p=0.002), and PTH (r=−0.67, p=0.006). In the OA group, DXA Z-scores showed a significant positive correlation only with dietary calcium intake (r=0.67, p=0.004). Vitamin D deficiency was common, with sufficiency (defined as>30 ng/mL) achieved in only 20% of GSD and 31.2% of OA patients.
Conclusion: Low bone mineral density is prevalent in both GSD and OA populations and appears to be influenced by modifiable factors such as calcium intake and vitamin D status. These findings highlight the importance of routine monitoring of bone health and nutrition in these patients. Multidisciplinary management is crucial for reducing long-term skeletal risks and optimizing clinical outcomes.
Keywords: bone mineral density, glycogen storage disease, organic acidemia, vitamin D
INTRODUCTION
Organic acidemias (OAs) and glycogen storage diseases (GSDs) are rare inherited metabolic disorders and may lead to long-term complications because of their multisystemic effects.1,2 Methylmalonic acidemia (MMA) and propionic acidemia (PA) are subtypes of OA caused by enzymatic defects in organic acid metabolism.3 MMA and PA result from the accumulation of metabolites due to impaired catabolism of valine, isoleucine, methionine, threonine, odd-chain fatty acids, and cholesterol.4 GSDs result from deficiencies in enzymes involved in glycogen metabolism, with type I and type III being the most prevalent subtypes.5,6 GSD type I results from glucose-6-phosphatase deficiency, while type III is due to a deficiency in the glycogen debranching enzyme.7
In hepatic forms of GSD, the primary therapeutic goal is the maintenance of normoglycemia. To achieve this, uncooked cornstarch is frequently used, alongside a diet restricted in simple sugars.8 Beyond normoglycemia, dietary management also aims to prevent complications, including hepatocellular adenomas/carcinomas, renal failure, myopathy, and osteoporosis.9 Dietary recommendations differ between subtypes: in GSDI, sucrose, fructose, galactose, and lactose are typically restricted, whereas in GSDIII, a diet rich in protein and/or fat with limited sucrose is often advised.5,6
In MMA and PA, dietary therapy focuses on minimizing the production of toxic organic acids while supporting normal growth and development. This is accomplished by restricting natural protein intake—particularly the precursor amino acids isoleucine, valine, threonine, and methionine—and supplementing with amino acid mixtures that exclude these compounds. Adequate energy intake is also essential to prevent catabolism.3,10
Monitoring and preserving bone health in these patient groups is critical not only for growth and development but also for maintaining quality of life and preventing fractures.11 Several studies have reported reduced bone mineral density (BMD) and increased fracture risk in patients with OA and GSD.12-15 Multiple factors influencing bone mineralization in these populations have been identified, including restrictive dietary regimens, insufficient calcium intake, chronic metabolic acidosis, persistent inflammation, vitamin D deficiency, mitochondrial dysfunction, and metabolic imbalances related to hypoglycemia in GSD.16,17
We hypothesized that low bone mineral density is common in patients with GSD and OA, and that it is associated with nutritional and biochemical parameters. This study was conducted to evaluate bone mineral density and investigate associated factors in patients with organic acidemias and glycogen storage disorders. The ultimate goal is to raise awareness of bone health monitoring in these patients and to contribute to the development of appropriate follow-up guidelines. There is a limited number of studies in the literature that have examined bone health and identified risk factors influencing BMD in these rare disorders. In particular, the scarcity of studies focusing on BMD in OA patients highlights the importance of this research in addressing a significant gap in the literature.
MATERIAL METHOD
Study design and subjects
A total of 31 patients were included in this study, comprising 15 individuals with GSD and 16 with OA. This study was conducted retrospectively at a single-center metabolic center. All participants had genetically confirmed diagnoses and were regularly followed at Gaziantep Cengiz Gökçek Maternity and Children’s Hospital . A retrospective design was chosen because these diseases are rare, and prospective records would be time-consuming to achieve a sufficient sample size. At our center, patients are regularly followed, and necessary disease-related assessments are recorded. This allowed us to obtain a sufficient dataset without creating additional patient burden on the hospital. The study protocol was approved by the Ethics Committee of Gaziantep University (Date: 24.07.2020, Approval Number: 2020/259), and written informed consent was obtained from all patients and/or their parents.
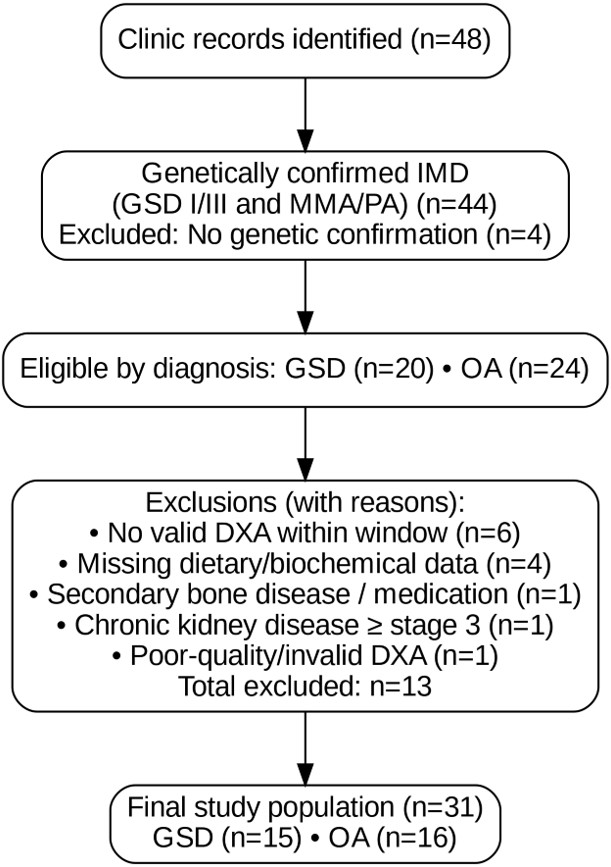
Inclusion criteria: Patients were eligible if they met the following: (i) a genetically confirmed diagnosis of GSD type I/III, or MMA/PA; (ii) age between 2 and 18 years at the time of the index DXA; (iii) at least one valid pediatric DXA scan with Z-scores reported according to the 2019 International Society for Clinical Densitometry (ISCD) recommendations; (iv) availability of a three-day dietary record analyzed with BeBIS 8.2, together with routine biochemical measurements (including vitamin D, PTH, calcium, phosphorus, and other relevant markers); and (v) ≥12 months of regular follow-up at our clinic (≥3 visits/year); (vi) Patients who had been attack-free within the past 12 months. Exclusion criteria: (i) Secondary bone disease unrelated to the underlying inborn metabolic disorder; (ii) chronic medications known to significantly affect bone metabolism in the 6 months prior to index DXA; (iii) chronic kidney disease ≥ stage 3; (iv) lack of contemporaneous dietary or laboratory data; (v) poor-quality or invalid DXA; (vi) Patients who experienced one or more metabolic decompensation episodes within the past 12 months. The process of patient selection, including inclusion and exclusion criteria, is summarized in a STROBE-compliant flow diagram (Figure 1).
Bone mineral density assessment
BMD measurements were performed using dual-energy X-ray absorptiometry (DXA) with a Hologic Explorer densitometer (USA). The device was calibrated daily using a standard phantom. Patients were positioned supine according to the 2019 pediatric guidelines of the ISCD. All scans were performed by the same experienced technician, thereby eliminating inter-observer variability. Device-reported quality control parameters remained within accepted standards. The precision error was evaluated in accordance with the recommendations of the ISCD, considering the minimum acceptable precision threshold for an individual technician.18 As per the 2019 ISCD official position, BMD results were reported as age-related Z-scores. A Z-score of ≤ −2.0 standard deviations (SD) was classified as “below the expected range for age”.18
Biochemical and anthropometric assessments
Medical records were reviewed to collect information on clinical status, dietary treatments, and relevant biochemical data. All patients had been monitored at least three times annually as part of their routine follow-up. No episodes of metabolic acidosis requiring hospitalization have been reported in the last year. All participants adhered to individualized dietary regimens tailored to their specific metabolic conditions. Normoglycemia for GSD patients was maintained with individualized dietary plans. Dietary compliance was assessed based on parent and patient reports at each visit, and patients were found to be compliant with the diet. Dietary intake was recorded over a three-day period and analyzed using the Nutrition Information System software (BeBIS 8.2) by a dietitian experienced in the nutritional management of metabolic disorders. BeBIS has been adapted for the Turkish population, and it is widely used in national nutrition research. Anthropometric measurements, including body weight and height, were assessed and converted into age- and sex-specific Z-scores using WHO Anthro and AnthroPlus software. Malnutrition was defined as a weight-for-age Z-score below −2 SD, while stunting was defined as a height-for-age Z-score below −2 SD. Biochemical analyses included measurements of serum calcium, phosphorus, alkaline phosphatase (ALP), parathyroid hormone (PTH), vitamin D, lactate, uric acid, creatine kinase (CK), triglycerides, aspartate aminotransferase (AST), alanine aminotransferase (ALT), bicarbonate, and pH. Vitamin D status with >30 ng/mL is considered sufficient.19 Information on physical activity, pubertal development, and menstrual status in female patients was not assessed.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 28.0. Descriptive statistics for continuous variables were presented as mean, minimum, maximum, and standard deviation (SD) values. The distribution of continuous variables was assessed using the Shapiro–Wilk test, histograms, and skewness–kurtosis values. As all variables showed normal distribution, parametric tests were applied. Associations between variables were examined using Pearson’s correlation coefficient. All correlations were performed with a single primary outcome (BMD Z-score). Therefore, multiple testing adjustment was not applied, as the analyses were designed to explore potential correlates of bone health rather than to test multiple independent hypotheses. Statistical significance was defined as a p-value <0.05.
RESULTS
Among the 15 patients diagnosed with GSD, 9 had GSDI (5 females, 4 males) and 6 had GSDIII (4 females, 2 males). The mean age of the GSD group was 7.15±3.54 years, ranging from 3.0 to 13.8 years. Of the 16 patients with OA, 12 had MMA (7 females, 5 males) and 4 had PA (2 females, 2 males), with a mean age of 7.53±4.17 years (range: 3.0–16.3 years). The mean duration of treatment was 6.77±3.34 years in the GSD group and 7.25±4.18 years in the OA group. The mean age and treatment duration of GSD and OA patients were similar. Table 1 provides detailed patient characteristics and DXA measurements.
| Table 1. Demographic characteristics and DXA Z-scores of patients with GSD and OA | ||
|---|---|---|
|
|
|
|
| Values are presented as mean ± standard deviation (min–max). GSD: Glycogen storage disease; OA: Organic acidemia; DXA: Dual-energy X-ray absorptiometry | ||
| Age (years) |
|
|
| Treatment duration (years) |
|
|
| Weight-for-age Z-score |
|
|
| Height-for-age Z-score |
|
|
| DXA Z-score |
|
|
Based on weight-for-age Z-scores, malnutrition was identified in 9.1% of GSD patients and 16.6% of OA patients. According to height-for-age Z-scores, 57.1% of GSD patients and 50% of OA patients were classified as stunted. The mean DXA Z-score was −2.59±1.45 in GSD patients, with 53.3% exhibiting reduced BMD. By subtype, 44.4% of GSDI and 66.6% of GSDIII patients had low BMD. In the OA group, the mean DXA Z-score was −1.91±1.19, and 50% of the patients had low BMD.
Biochemical parameters of the patients are presented in Table 2. Serum levels of calcium, phosphorus, ALP, and PTH were within normal ranges in both groups. However, the mean serum vitamin D levels were below the sufficiency threshold in both patient groups. Only 20% of GSD patients (3 out of 15) and 31.2% of OA patients (5 out of 16) had sufficient vitamin D levels (>30 ng/mL). Elevated mean serum lactate levels were observed in GSDI and OA patients, while GSDIII patients exhibited elevated CK levels. Additionally, GSD patients had elevated mean triglyceride, AST, and ALT levels. In OA patients, bicarbonate levels were within normal limits. Dietary calcium intake, expressed as a percentage of the recommended daily allowance, was notably higher in OA patients compared to those with GSD. Dietary calcium intake as a percentage of the recommended daily intake was significantly higher in OA patients compared to patients with GSD.
| Table 2. Biochemical parameters and calcium intake levels of patients with GSD and OA | |||
|---|---|---|---|
| Parameters |
|
|
|
| Values are presented as mean ± standard deviation (min–max). Reference ranges represent clinical normal values. ALP: Alkaline phosphatase; PTH: Parathyroid hormone; CK: Creatine kinase; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase | |||
| Calcium, mg/dL |
|
|
|
| Phosphorus, mg/dL |
|
|
|
| ALP, U/L |
|
|
|
| PTH, pg/mL |
|
|
|
| Vitamin D, ng/mL |
|
|
|
| Lactate, mmol/L (GSDI) |
|
|
|
| Uric acid, mg/dL (GSDI) |
|
|
|
| CK, U/L (GSDIII) |
|
|
|
| Triglyceride, mg/dL |
|
|
|
| AST, U/L |
|
|
|
| ALT, U/L |
|
|
|
| Bicarbonate, mEq/L |
|
|
|
| Ph (GSDI) |
|
|
|
| Calcium intake (% of requirement met) |
|
|
|
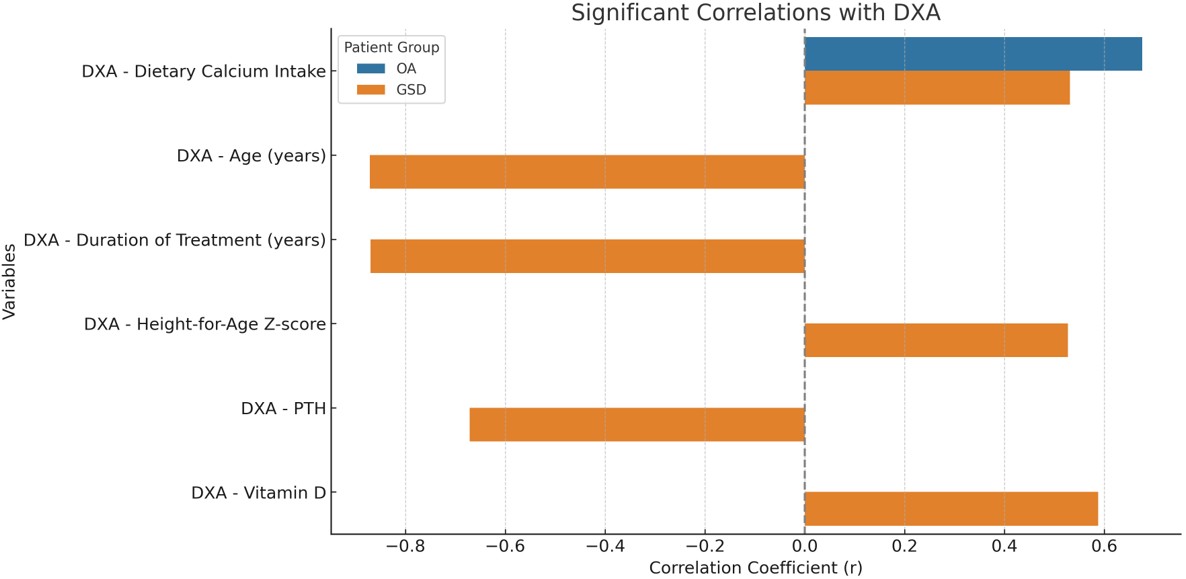
Variables showing significant correlations with patients’ DXA scores are presented in Figure 2. A significant positive correlation was found between dietary calcium intake and DXA Z-scores in both GSD and OA patients (p<0.05). In the GSD group, DXA Z-scores also showed a significant positive correlation with height-for-age Z-scores and serum vitamin D levels, and a significant negative correlation with age, treatment duration, and serum PTH levels (p<0.05). In addition, no significant association was found between lactate levels and DXA measurements in patients with GSDI and OA (p>0.05).
DISCUSSION
This study aimed to evaluate bone health and the factors influencing BMD in patients with OA and GSD. The findings demonstrated a high prevalence of reduced BMD in both patient groups. In particular, among GSD patients, BMD was significantly associated with dietary calcium intake, age, duration of treatment, height-for-age Z-score, serum PTH levels, and serum vitamin D status. There are studies in the literature with limited samples evaluating bone health in GSD and OA patients.12,15,20
In our study, malnutrition was identified in 9.1% of GSD patients and 16.6% of OA patients. Stunting was identified in 57.1% of GSD patients and 50% of OA patients. One study found a 66% rate of stunting and a 5.5% rate of underweight in GSD patients.21 In a similar study in the literature, the stunting rate in GSD patients was reported as 50%.22 Consistent with our findings, previous studies have also reported a high prevalence of stunting and malnutrition among OA patients.10 Consistent with these findings, we observed a high rate of stunting, particularly among GSD patients. Given the observed associations between anthropometric parameters and DXA scores, routine monitoring of growth status in these patients is essential.
When the DXA results of the patients were evaluated, decreased BMD was observed in 53.3% of GSD patients. This high prevalence is consistent with previous studies and supports the notion that GSD patients are at significant risk for compromised bone health. Contributing factors include recurrent hypoglycemia, vitamin D deficiency, and suboptimal metabolic control. In GSDIII patients, the pathophysiology of low BMD is likely multifactorial, involving altered muscle physiology, metabolic dysregulation, and nutritional inadequacies. Additionally, physical inactivity and associated muscle weakness may further exacerbate bone loss.5,23,24 Several studies have reported an increased risk of osteoporosis and fragility fractures in patients with GSD5,6,12-14,22,24-27 particularly in those with GSDIII.26 A recent Polish cohort likewise demonstrated predominantly negative BMD Z-scores, emphasizing the need for careful dietary and metabolic monitoring21. Although treatment guidelines for GSD emphasize the need for DXA assessments5,6, they provide no detailed recommendations regarding the timing or frequency. Our results indicate that bone health evaluation should be initiated at an earlier stage in these patients. Also, these findings underscore the importance of early detection and continuous follow-up to prevent skeletal complications. Identifying factors that influence BMD may enhance patient management and guide clinical decisions.
In the OA group, reduced BMD was observed in 50% of patients, consistent with previous reports suggesting a high prevalence of impaired bone health in this population. Potential contributing factors include protein-restricted diets, chronic metabolic acidosis, and vitamin D deficiency.15 A case series involving OA patients similarly reported decreased BMD in the majority of participants.28 In a study involving MMA patients, 54.5% of patients were found to have decreased bone mineral density.15 Although current treatment guideline for OA recommend performing DXA assessments in older children2, our findings demonstrate that low bone mineral density can already be present at younger ages. These results suggest that bone health evaluation should be initiated earlier in this patient group. The literature on bone health in OA is extremely limited, with only a few small studies and case series available. This scarcity of data makes our findings particularly valuable, as they expand the limited evidence base and provide new insights into skeletal outcomes in MMA and PA. Given the chronic nature of protein restriction and cumulative metabolic derangements in these disorders, our results emphasize the need for systematic bone health monitoring and support multicenter longitudinal studies to better define risk factors and long-term outcomes in this neglected group. The results of our study are in line with these limited reports and support the need for routine bone health assessments and individualized monitoring strategies in patients with OA.
In our study, it was observed that the majority of GSD and OA patients had insufficient serum vitamin D levels. A study reported that 78.8% of GSD patients had low serum vitamin D levels.21 Another study reported vitamin D insufficiency in 84% of GSD patients.27 Moreover, one study showed a significant positive correlation between serum vitamin D levels and DXA Z-scores, which was also observed in our cohort.14 Both GSD and OA patients require regular monitoring of serum parameters and careful monitoring with the use of supplements such as vitamin D when necessary. Similar to the literature, serum calcium, phosphorus, ALP, and PTH levels in our GSD patients are within normal values.21,27,29 Similar to the studies conducted, serum triglyceride, AST, ALT, and CK levels for GSDIII patients were found to be higher than normal in the patients included in the study.12,13,20 In our GSDI patients, uric acid levels were found to be higher than normal. High uric acid levels are among the complications of the disease in GSDI patients6 and uric acid levels were higher than normal in the GSDI patients included in our study. One study has reported that serum lactate levels tend to be low in most patients with GSDI.21 In GSDI, impaired urate clearance is a known complication; however, no significant correlation between uric acid and BMD was observed in our cohort. This likely reflects the multifactorial nature of bone involvement in GSD, where chronic acidosis, hypoglycemia, vitamin D deficiency, and dietary restrictions may outweigh the effect of uric acid. The paradoxical role of uric acid—protective at moderate levels but detrimental when chronically elevated—further complicates interpretation.30 This difference may be due to various factors, including the clinical stability of the patients included in the study, their adherence to treatment, and the diversity of dietary treatments.
Regarding calcium intake, the percentage of recommended daily intake met was 81.2% overall in the GSD group (83.1% in GSDI and 78.5% in GSDIII). Regarding calcium intake, the percentage of recommended daily intake met was 161.8% in the OA group. In a similar study in the literature, it was stated that the vast majority of GSD patients (94% of patients) had inadequate calcium intake.21 In line with our results, Jacoby et al.16 also highlighted the impact of nutritional factors on bone health in hepatic GSD patients. GSDI patients are advised to follow a diet limited to dairy products containing nutrients such as calcium, phosphorus, and protein that will support bone health.6 However, lactose-free enteral formulas added to the diet may compensate for this deficiency. Similarly, OA patients, who receive amino acid mixtures free of precursor amino acids, often consume high levels of calcium from these formulas. In both patient groups, a positive and significant correlation was observed between dietary calcium intake and DXA scores (p<0.05). The bioavailability of calcium from foods is higher than that of calcium from amino acid mixtures.31 Therefore, even if OA patients consume high amounts of calcium in their diets, their bodily absorption may be limited. These results suggest that the amount of calcium patients consume may have an impact on bone mineralization. Therefore, even if OA patients consume large amounts of calcium through amino acid mixtures, their absorption may remain limited. Our findings indicate that calcium intake is a critical determinant of bone mineralization. In GSD patients, dietary calcium intake should be actively supported, whereas in OA patients, where the bioavailability of calcium from amino acid mixtures is relatively low, increasing natural protein intake—when clinically tolerated—may provide more effective support for skeletal health.
This study has several limitations. Due to the rarity of the disease, this study was conducted on a small and heterogeneous patient group. Furthermore, there is no information on the patients’ physical activity levels, pubertal status, or menstrual status in female patients. A further limitation is the limited availability of published data on OA-related bone health, which constrains meaningful comparisons with existing studies. This underscores both the novelty and the importance of our findings. Further longitudinal studies with larger sample sizes are needed to better understand the determinants of bone health in these populations.
CONCLUSIONS
This study demonstrates that low bone mineral density is common among patients with GSD and OA. In these individuals, impaired bone mineralization may increase the long-term risk of osteoporosis. Existing guidelines for GSD and OA highlight the importance of DXA assessments but lack specific recommendations on their timing and frequency. The present study demonstrates that low BMD can be detected even in younger patients, underscoring the importance of initiating bone health monitoring at earlier stages. Our findings emphasize the need for early and comprehensive monitoring, particularly during childhood and adolescence. Clinical follow-up should include DXA scans, biochemical evaluations, nutritional assessments, and growth monitoring. A multidisciplinary follow-up approach involving metabolic specialists, dietitians, and physiotherapists is essential for optimal patient care. Early and regular individualized follow-up strategies may help to prevent future skeletal complications and improve overall quality of life.
Ethical approval
This study has been approved by the Ethics Committee of Gaziantep University (approval date 24.07.2020, number 2020/259). Written informed consent was obtained from the participants.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Gümüş E, Özen H. Glycogen storage diseases: an update. World J Gastroenterol. 2023;29:3932-63. https://doi.org/10.3748/wjg.v29.i25.3932
- Manoli I, Sloan JL, Venditti CP. Isolated methylmalonic acidemia. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews®. Seattle (WA): University of Washington; 2022.
- Fraser JL, Venditti CP. Methylmalonic and propionic acidemias: clinical management update. Curr Opin Pediatr. 2016;28:682-93. https://doi.org/10.1097/MOP.0000000000000422
- Saleemani H, Egri C, Horvath G, Stockler-Ipsiroglu S, Elango R. Dietary management and growth outcomes in children with propionic acidemia: a natural history study. JIMD Rep. 2021;61:67-75. https://doi.org/10.1002/jmd2.12234
- Kishnani PS, Austin SL, Arn P, et al. Glycogen storage disease type III diagnosis and management guidelines. Genet Med. 2010;12:446-63. https://doi.org/10.1097/GIM.0b013e3181e655b6
- Kishnani PS, Austin SL, Abdenur JE, et al. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014;16:e1. https://doi.org/10.1038/gim.2014.128
- Laforêt P, Weinstein DA, Smit GPA. The glycogen storage diseases and related disorders. In: Saudubray JM, van den Berghe G, JHW, editors. Inborn metabolic diseases: diagnosis and treatment. Springer Berlin Heidelberg; 2012: 115-40. https://doi.org/10.1007/978-3-642-15720-2_6
- Ross KM, Ferrecchia IA, Dahlberg KR, Dambska M, Ryan PT, Weinstein DA. Dietary management of the glycogen storage diseases: evolution of treatment and ongoing controversies. Adv Nutr. 2020;11:439-46. https://doi.org/10.1093/advances/nmz092
- Steunenberg TAH, Peeks F, Hoogeveen IJ, et al. Safety issues associated with dietary management in patients with hepatic glycogen storage disease. Mol Genet Metab. 2018;125:79-85. https://doi.org/10.1016/j.ymgme.2018.07.004
- Dixon M, MacDonald A, White FJ. Disorders of amino acid metabolism, organic acidaemias and urea cycle disorders. In: Shaw V, editor. Clinical paediatric dietetics. John Wiley & Sons, Ltd; 2020: 513-98. https://doi.org/10.1002/9781119467205.ch28
- Langeveld M, Hollak CEM. Bone health in patients with inborn errors of metabolism. Rev Endocr Metab Disord. 2018;19:81-92. https://doi.org/10.1007/s11154-018-9460-5
- Melis D, Rossi A, Pivonello R, et al. Reduced bone mineral density in glycogen storage disease type III: evidence for a possible connection between metabolic imbalance and bone homeostasis. Bone. 2016;86:79-85. https://doi.org/10.1016/j.bone.2016.02.012
- Kaiser N, Gautschi M, Bosanska L, et al. Glycemic control and complications in glycogen storage disease type I: results from the Swiss registry. Mol Genet Metab. 2019;126:355-61. https://doi.org/10.1016/j.ymgme.2019.02.008
- Minarich LA, Kirpich A, Fiske LM, Weinstein DA. Bone mineral density in glycogen storage disease type Ia and Ib. Genet Med. 2013;14:737-41. https://doi.org/10.1038/gim.2012.36
- Emam M, Sedafy HE, Hussain Omar O, Hammad N, Elnabi MH, Elsayed SM. Bone health assessment in patients with methylmalonic acidemia. QJM An Int J Med. 2024;1:117. https://doi.org/10.1093/qjmed/hcae175.317
- Jacoby JT, Bento Dos Santos B, Nalin T, et al. Bone mineral density in patients with hepatic glycogen storage diseases. Nutrients. 2021;13:2987. https://doi.org/10.3390/nu13092987
- Forny P, Hörster F, Ballhausen D, et al. Guidelines for the diagnosis and management of methylmalonic acidaemia and propionic acidaemia: first revision. J Inherit Metab Dis. 2021;44:566-92. https://doi.org/10.1002/jimd.12370
- ISCD. 2019 ISCD official positions pediatric. Available at: https://iscd.org/learn/official-positions/pediatric-positions/ (Accessed on Jul 23, 2025)
- Corsello A, Spolidoro GCI, Milani GP, Agostoni C. Vitamin D in pediatric age: current evidence, recommendations, and misunderstandings. Front Med (Lausanne). 2023;10:1107855. https://doi.org/10.3389/fmed.2023.1107855
- Melis D, Pivonello R, Cozzolino M, et al. Impaired bone metabolism in glycogen storage disease type 1 is associated with poor metabolic control in type 1a and with granulocyte colony-stimulating factor therapy in type 1b. Horm Res Paediatr. 2014;81:55-62. https://doi.org/10.1159/000351022
- Seliga EE, Hajdacka M, Jaworski M, et al. Nutritional status of patients with glycogen storage diseases - polish experience. http://Prerpints.org; 2023. Available at: https://www.preprints.org/manuscript/202309.0248/v1
- Eminoğlu FT, Tümer L, Okur İ, Ezgü FS, Hasanoğlu A. Clinical properties and disease prognosis in cases of glycogen-storage disease type 1a and type 1b. Turkish Arch Pediatr. 2013;48:117-22. https://doi.org/10.4274/tpa.185
- Dagli A, Sentner CP, Weinstein DA. Glycogen storage disease type III. GeneReviews®. Seattle: University of Washington; 2010.
- Hijazi G, Paschall A, Young SP, et al. A retrospective longitudinal study and comprehensive review of adult patients with glycogen storage disease type III. Mol Genet Metab Rep. 2021;29:100821. https://doi.org/10.1016/j.ymgmr.2021.100821
- Rossi A, Simeoli C, Pivonello R, et al. Endocrine involvement in hepatic glycogen storage diseases: pathophysiology and implications for care. Rev Endocr Metab Disord. 2024;25:707-25. https://doi.org/10.1007/s11154-024-09880-2
- Sentner CP, Hoogeveen IJ, Weinstein DA, et al. Glycogen storage disease type III: diagnosis, genotype, management, clinical course and outcome. J Inherit Metab Dis. 2016;39:697-704. https://doi.org/10.1007/s10545-016-9932-2
- Javadi SH, Farina A. A high prevalence of low bone mineral density in children with glycogen storage disease type III. Arch Med Sci. 2020;131:793-9.
- Touati G, Valayannopoulos V, Mention K, et al. Methylmalonic and propionic acidurias: management without or with a few supplements of specific amino acid mixture. J Inherit Metab Dis. 2006;29:288-98. https://doi.org/10.1007/s10545-006-0351-7
- Vai S, Falchetti A, Corbetta S, et al. Glycogen storage disease type I and bone: clinical and cellular characterization. Calcif Tissue Int. 2024;115:661-72. https://doi.org/10.1007/s00223-024-01302-4
- Zhang J, Sun N, Zhang W, et al. The impact of uric acid on musculoskeletal diseases: clinical associations and underlying mechanisms. Front Endocrinol (Lausanne). 2025;16:1515176. https://doi.org/10.3389/fendo.2025.1515176
- Rondanelli M, Faliva MA, Barrile GC, et al. Nutrition, physical activity, and dietary supplementation to prevent bone mineral density loss: a food pyramid. Nutrients. 2021;14:74. https://doi.org/10.3390/nu14010074
Copyright and license
Copyright © 2025 The author(s). This is an open-access article published by Aydın Pediatric Society under the terms of the Creative Commons Attribution License (CC BY) which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.